Ovarian Carcinoma:
General:
Ovarian
carcinoma is the second most common tumor of the female genital
tract after carcinoma of the endometrium [21]. Ovarian carcinoma
is the leading cause of death among women with gynecologic
malignancies [11,21,27]. This is because patients
commonly present with advanced disease (about 75% of cases [24])
due to a lack of symptoms in the early stages which results in a
5 year survival rate of only 46% [16].
Survival
is mostly dependent on disease stage [26]. The 5 year survival
for stage I disease is 70-90% (disease confined to the ovaries),
but survival for stage III and higher is only 30% (and 15% for
stage IV disease) [23,24,26]. Metastases are present in
two-thirds of patients at the time of presentation. Metastatic
disease occurs most commonly to the intraperitoneal surfaces of
the bowel and opposite ovary [7,17]. The
flow of peritoneal fluid from the pelvis to the upper abdomen
and gravity promote deposition of malignant cells typically to
the subdiaphragmatic surfaces and pelvic peritoneum, with
preferential flow to the right [29]. The presence of
peritoneal deposits in the pelvis confers stage IIB disease,
whereas spread to the upper abdomen is considered stage III
[29]. Metastases can also spread via lymphatics to para-aortic
and peritoneal lymph nodes, and, least commonly, hematogenously
[7,17].
Primary surgical debulking of all sites of disease is very important for patients to receive a survival benefit [16]. However, even in tertiary centers using aggressive surgical approaches, complete cytoreduction can be achieved in only about one-third of patients [29]. This is because certain sites in the peritoneum are challenging to access, such as the porta hepatis or the presence of extensive serosal or mesenteric implants [29]. Cytoreductive surgery is followed by paclitaxel and platinum-based cytotoxic chemotherapy [24].
Imaging of ovarian carcinoma requires meticulous exam technique. Some authors suggest the use of bladder lavage with a two way catheter during image acquisition to decrease artifacts from the urinary bladder due to renal excretion of the agent [4,7].
The addition of FDG PET imaging to conventional CT imaging in patients with ovarian carcinoma results in improved staging accuracy [16]. In one study, for lesions outside the pelvis, sensitivity improved from 24% to 63% when FDG images were used in conjunction with the CT exam [16]. Overall post-operative staging improved from 53% to 87% [16].
PET imaging in the evaluation of the primary
ovarian tumor:
FDG-PET
performance in the identification of primary ovarian malignancy
has been mixed with reported sensitivities between 52-58%, and
specificities between 76-78% [22]. False-positive results have
been reported in association with inflammatory adenexal masses [5,
7, 9], corpus luteum cysts [9], endometriomas (up to 22% of
endometriomas) [9], dermoid cyst [21], teratoma [21], other benign
ovarian tumors [9], and adjacent physiologic urinary tract and
gastrointestinal activity [9]. During imaging for ovarian
carcinoma, some authors suggest the use of bladder lavage with a
two-way catheter during image acquisition to decrease artifacts
due to urinary tract activity [7].
False-negative
exams
can occur in borderline tumors with low malignant potential and
in early stage tumors [5, 9]. Overall sensitivity has been
reported to be 58%, specificity 76%, positive predictive value
25%, negative predictive value 93%, and accuracy 74% [9]. The
low sensitivity may be related to the often cystic nature of the
tumors which contain only small solid components [16].
Lymph node metastases in ovarian carcinoma:
Lymph
node involvement at the time of initial surgery has a reported
incidence of 25% in patients with stage I disease; 50% in stage
II, and 74& in stages III and IV [24]. For the detection of
lymph node metastases FDG-PET has a reported sensitivity of 73%,
specificity of 92%, a positive predictive value of 80, a
negative predictive value of 89%, and an accuracy of 86% [5].
Distant
metastases:
Hematogenous
dissemination of disease at the time of diagnosis is uncommon-
only 2-3% of patients are found to have pulmonary or hepatic
involvement [24]. Pleural effusion is the most common finding of
stage IV disease, followed by liver metastases [24].
|
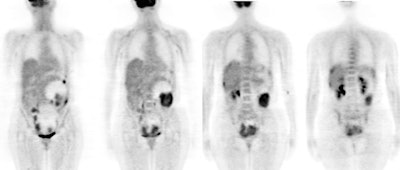
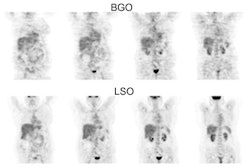
Ovarian cancer: The patient shown below was a 56 year old female who complained of severe abdominal pain. Based upon physical exam ovarian carcinoma was suspected. The patient's CA-125 level was elevated. PET imaging revealed extensive intra-abdominal and pelvic disease including capsular liver metastases. Case courtesy of Northern California PET Imaging Center and CTI. |
|
|
Recurrent
disease:
Early detection of recurrent disease is essential for effective second-line therapy [5]. Between 40%-60% of patients will have an intra-abdominal relapse usually associated with peritoneal carcinomatosis [5]. The treatment for recurrent ovarian cancer depends upon the tumor bulk and extent [18]. Because there is significant morbidity associated with surgical intervention, proper identification of all sites of disease is critical to patient management [18]. For patients in whom recurrence is diagnosed, a survival benefit is seen when cytoreductive surgery is optimal [18]. Secondary cytoreductive surgery is beneficial if the largest deposit is less than 10 cm and there is no gross tumor after surgery [17].
CA-125
is a serum marker used to monitor patients for recurrence. Between
95%-100% of patients with an elevated serum CA-125 are found to
have recurrence [6]. Unfortunately, CA-125 levels may be falsely
negative in 25% to 50% of patients with recurrence [6,12].
Additionally, CA-125 does not provide information about the site
of recurrence [8].
It is
well established that anatomic imaging modalities fail to
adequately identify recurrent ovarian carcinoma. In the detection
of recurrent ovarian carcinoma, CT has a reported sensitivity and
specificity of 40%-63% and 81%-83%, respectively [6, 10].
Sensitivity for implants is generally greater than 50% for most
sites except the small bowel and mesentery where lesions can be
difficult to appreciate due to volume averaging [17]. Second-look
surgery is considered the standard to assess for recurrence
(between 36-73% of patients may have persistent disease detected
at second-look laparotomy [21], but it is an invasive procedure
[6, 7]. Additionally, second-look surgery is not perfect for
detecting all small lesions within the abdomen and it is of no
help in detecting distant metastases [8]. In fact, recurrent
ovarian carcinoma occurs in 20%-50% of patients even after a
negative second-look surgery [10,19].
FDG-PET
imaging can be a complementary imaging modality for the evaluation
of recurrent ovarian carcinoma, particularly when conventional
imaging is inconclusive or negative in patients with abnormal
CA125 levels [8,12,15]. Evidence of recurrent ovarian cancer on
PET exams can precede conventional exam (CT) findings by a median
of 6 months [14,24].
Omental
carcinomatosis:
The sensitivity of CT in detecting peritoneal metastases
ranges from 64-93%, with specificity of 92-100% [29]. Contrast
enhanced MRI has reported superior sensitivity to CT (from
85-90%) [29]. Peritoneal carcinomatosis displays restricted
diffusion and increased lesion conspicuity on DWI imaging [29].
Omental
carcinomatosis
can also be identified on FDG PET-CT and PET-MRI imaging,
however, PET is limited by its spatial resolution, which limits
detection of small peritoneal deposits, and physiologic bowel
activity which can mask serosal lesions [29]. Findings on PET
imaging which suggest the diagnosis of peritoneal carcinomatosis
include: 1- uniform low-grade tracer uptake throughout the
abdomen obscuring the normal discrete visceral outlines of the
bowel and liver; and 2- focal nodular areas of tracer uptake
[3].
For
the detection of peritoneal carcinomatosis, FDG-PET-CT has a
reported sensitivity of 60-71% (other authors report 80-100%
[29]), specificity of 100%, positive predictive value of 100%,
negative predictive value of 76%, and an accuracy of 85% [5,28].
FDG PET imaging is complementary to conventional imaging and the
combination of the two modalities increases the overall
detection of peritoneal carcinomatosis, the detection of
peritoneal disease is also better by PET/CT (sensitivity 84%)
[3,28]. PET-MRI has been shown to have high diagnostic accuracy
for the detection of peritoneal carcinomatosis (97%), with a
sensitivity of 80% and a specificity of 100% [29]. False
negative exams can occur with small volume and microscopic
disease [3]. Mucinous lesion histology which is known to only
demonstrate low metabolic activity can also result in false
negative imaging [29].
The implementation of PET imaging in the evaluation of recurrent or residual ovarian cancer can help to decrease health care costs by decreasing unnecessary second look laparotomies [21]. The positive predictive value is about 93%, and PET has been shown to be superior to CT and MR in the detection of recurrent disease [21]. PET findings have been shown to lead to a decrease in laparotomies from 70% to 5% of patients, and 35% of patients underwent less invasive laparoscopy [21].
In patients with recurrent ovarian carcinoma the number and SUVmax of peritoneal tumor deposits is associated with poor survival [23].
|
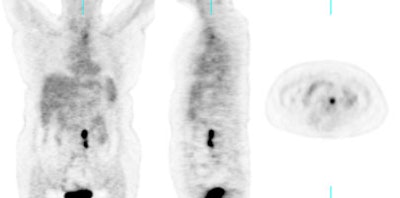
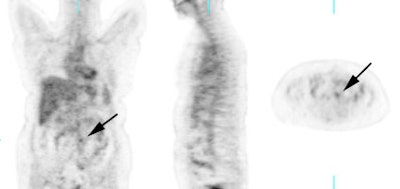
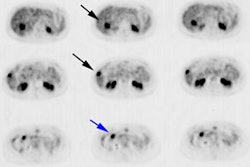
Recurrent ovarian cancer: 58 year old female with a history of treated recurrent ovarian carcinoma. Follow-up CT scan showed a left para-aortic soft tissue mass which had remained stable for 3 months. PET FDG exam revealed uptake of tracer in the left para-aortic mass, compatible with metastatic retroperitoneal lymphadenopathy (top row of images). Radiation therapy was instituted and a follow-up PET exam after completion of therapy revealed marked interval decrease in tracer uptake (black arrow). There is some faint residual uptake evident. Case courtesy North Texas Clinical PET Institute and CTI. |
|
|
Response
to therapy:
Using
a decrease in SUV of 20% compared to baseline after the first
cycle of chemotherapy is associated with improved overall
survival (38.3 months compared to 23.1 months in patients who
demonstrate no metabolic response [24].
Patient
Management:
Results of the FDG-PET exam can provide additional information in up to 58% of patients with suspected recurrent ovarian cancer and these findings can impact on patient management in about 40% of cases [8]. Patients with a negative PET scan have been shown to have a longer relapse-free interval than patients with a positive PET scan [14].
Limitations
of FDG Imaging in Ovarian Carcinoma:
FDG-PET imaging is limited in the detection of disseminated lesions smaller than 1 cm [8, 10]. Physiologic activity in the abdomen can lower the specificity of the PET exam [17]. Renal and urinary activity can mask lesions [17]. False-positive exams can occur and conventional imaging of all areas of abnormal FDG accumulation should be performed to confirm a pathologic lesion.
REFERENCES:
(3) J Nucl Med 2003; Turlakow A, et al. Peritoneal carcinomatosis: role of 18F-FDG-PET. 44: 1407-1412
(4) J Nucl Med 2003; Koyama K, et al. Evaluation of 18F-FDG PET with bladder irrigation in patients with uterine and ovarian tumors. 44: 353-358
(5) Int J Gynecol Cancer 1999; Schroder W, et al. The role of 18F-fluoro-deoxyglucose positron emission tomography (18F-FDG PET) in diagnosis of ovarian cancer. 9: 117-122
(6) J Reprod Med 1999; Yuan CC, et al. Whole-body PET with (Fluorine-18)-2-deoxyglucose for detecting recurrent ovarian carcinoma. Initial report. 44: 775-778
(7) Eur Radiol 2000; Kubik-Huch RA, et al. Value of (18F)-FDG positron emission tomography, computed tomography, and magnetic resonance imaging in diagnosing primary and recurrent ovarian carcinoma. 10: 761-67
(8) AJR 2001; Nakamoto Y, et al. Clinical value of positron emission tomography with FDG for recurrent ovarian cancer. 176: 1449-54
(9) Radiology 2002; Fenchel S, et al. Asymptomatic adenexal masses: correlation of FDG PET and histopathologic findings. 223: 780-788
(10) AJR 2002; Cho SM, et al. Usefulness of FDG PET for assessment of early recurrent epithelial ovarian cancer. 179: 391-395
(11) AJR 2001; Dachman AH, et al. Role
of chest CT in the follow-up of ovarian adenocarcinoma. 176:
701-705
(12) Eur J Nucl Med Mol Imaging 2002;
Torizuka T, et al. Ovarian cancer recurrence: role of
whole-body positron emission tomography using
2-[fluorine-18]-fluoro-2-deoxy- D-glucose. 29: 797-803
(13) Anticancer Res 2001; Yen RF, et al.
Whole body positron emission tomography with 18F-fluoro-2-deoxyglucose
for the detection of recurrent ovarian cancer. 21: 3691-94
(14) Gynecol Oncol 2001; Zimny M, et al.
2-[Fluorine-18]-fluoro-2-deoxy-d-glucose positron emission
tomography in the diagnosis of recurrent ovarian cancer. 83:
310-315
(15) Clin Positron Imaging 2000;
Jimenez-Bonilla J, et al. Clinical impact of 18F-FDG-PET
in the suspicion of recurrent ovarian carcinoma based on elevated
tumor marker serum levels. 3: 231-236
(16) AJR 2004; Yoshida Y, et al. Incremental benefit of FDG positron emission tomography over CT alone for the preoperative staging of ovarian cancer. 182: 227- 233
(17) Radiographics 2004; Pannu HK, et al. PET-CT in recurrent ovarian cancer: initial observations. 24: 209-223
(18) AJR 2004; Funt SA, et al. Role of CT in the management of recurrent ovarian cancer. 182: 393-398
(19) Radiology 2004; Sironi S, et al. Integrated FDG PET/C in patients with persistent ovarian cancer: correlation with histologic findings. 233: 433-440
(20) Radiol Clin N Am 2004; Kumar R, Alavi A. PET imaging in gynecologic malignancies. 42: 1155-1167
(21) J Nucl Med 2005; Pandit-Taskar N. Oncologic imaging in gynecologic malignancies. 46: 1842-1850
(22) AJR 2010; Iyer VR, Lee SI. MRI, CT, and PET/CT for ovarian cancer detection and adenexal lesion characterization. 194: 311-321
(23) Radiology 2010; Sala E, et al. Recurrent ovarian cancer: use of contrast-enhanced CT and PET/CT to accurately localize tumor recurrence and to predict patients' survival. 257: 125-134
(24) Radiographics 2011; Son H, et al. Role of FDG PET/CT in staging of recurrent ovarian cancer. 31: 569-583
(25) Radiology 2011; Lutz AM, et al. Early diagnosis of ovarian carcinoma: is a solution in sight? 259: 329-345
(26) Radiographics 2011; Lalwani N, et al. Histologic, molecular,
and cytogenic features of ovarian cancers: implications for
diagnosis and treatment. 31: 625-646
(27) J Nucl Med 2015; Lee SI, et al. Evaluation of gynecologic
cancer with MR imaging, 18F-FDG PET/CT, and PET/MR
imaging. 56: 436-443
(28) Radiographics 2017; Lakhani A, et al. FDG PET/CT pitfalls in
gynecoogic and genitourinary oncologic imaging. 37: 577-594
(29) AJR 2020; Lee EYP, et al. Molecular imaging of peritoneal
carcinomatosis. 215: 305-312