BOSTON - Tucked into countless virtual colonoscopy papers and presentations over the years has been a ubiquitous, almost apologetic statement noting that reporting standards have yet to be developed for CT colonography. Leading researchers in the field believed they needed more time to evaluate the rapidly accumulating evidence in the virtual colonoscopy, gastroenterology, and pathology literature.
The disclaimer took a step toward obsolescence at this year's International Symposium on Virtual Colonoscopy, however, when a working group of published VC researchers outlined new recommendations aimed at defining virtual colonoscopy findings and patient management criteria.
In collaboration with the American College of Radiology's Colon Cancer Committee and other organizations, the CT Colonography Reporting and Data System (C-RADS) working group that began at last year's symposium has produced a set of evidence-based recommendations along the lines of the successful BI-RADS classifications used in breast imaging.
The C-RADS guidelines, which are expected to be published in the coming months and embraced by the Reston, VA-based American College of Radiology (ACR) in a similar form next spring, do not tell radiologists how to perform the virtual exam or optimize their results.
But the evidence-based interpretation tables should go a long way toward calming a kaleidoscope of approaches that have characterized VC reporting and follow-up since its inception a decade ago. Dr. Michael Zalis, associate professor of radiology at Massachusetts General Hospital in Boston, offered an overview in a talk on Thursday.
C-RADS will help manage patients, providing a common set of terms for VC findings, and making it easier to compare studies performed at different sites, he said.
"It's easy to imagine nowadays as the number of VC studies is increasing: a patient having a study at one site will then go to another center," Zalis said. "(With C-RADS) we can evaluate reports in a more time-efficient fashion, and it will facilitate the large-scale analysis of our experience with VC. This is something that third-party payors, the federal government of the U.S., and indeed our own (medical) societies are going to require us to perform."
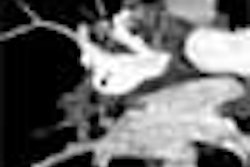
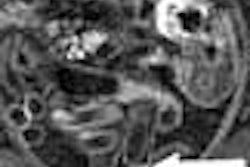
The guidelines are based on the premise that the target lesion in virtual colonoscopy is the advanced adenoma following the established adenoma-carcinoma sequence.
"The advanced adenoma ... is usually defined as a lesion 1 cm or greater in size, containing frank-grade dysplasia and/or villous histology," Zalis said. "At least until we have real evidence to show otherwise, hyperplastic polyps are really not relevant for screening. Even when we talk about an alternative (mutagenic) pathway arising from a hyperplastic polyp, the real lesion we're trying to detect is still an (advanced) adenoma."
| Table 1 covers feature descriptions of the target lesion, including size ("Single largest dimension of polyp head (excluding stalk if present) in either (stated) MPR or 3D views") defined as 1-5 mm, 6-9 mm, and ≥ 10 mm. The morphology is reported as sessile, pedunculated, or flat; the location specified among the six standard segmental divisions of the colon; and the attenuation is defined as soft-tissue or fat. |
The second principle is that polyps grow slowly, nearly always requiring a decade or more to reach an advanced stage, Zalis said. In colonoscopy, precancerous precursor lesions are resected most often.
"Unlike in the case of lung-cancer screening or breast-cancer screening, we should not expect that the target lesion we will identify is a malignancy in the vast majority of cases," Zalis said. "That is fortunate for us because it makes our job easier."
Size is the most important criteria from a radiological standpoint, and forms the basis of most of the management recommendations:
Polyps smaller than 5 mm should not be reported. Normal colorectal screening intervals should be continued. The vast majority of such lesions are not only hyperplastic, but chances are exceedingly small that a single subcentimeter polyp represents the screening target, Zalis said.
Polyps 6-9-mm in size should be reported. The follow-up for such lesions should be individualized, but might reasonably occur within three years by either well-performed VC or endoscopy.
"All of the recommendations we make here are guidelines that need, ultimately, to be individualized to a particular patient and a particular practice, in a particular situation," he said. "So when we talk about roughly a three- or three- to five-year follow-up for lesions of this size category, we have to understand that there's going to be some variability depending on the age. For example, in an older patient we may consider a longer interval for surveillance. In a younger patient, where there's a high degree of confidence on the part of the reader, and for a lesion that is closer to the upper limit of this size category, we may consider a shorter interval."
| Table 2 includes lesion classification and suggested follow-up. Classifications include C0, "Inadequate Study/Awaiting Prior Comparisons" to account for inadequate prep or insufflation in specified colonic segments, indicating that lesions ≥ 10 mm cannot be excluded, or that the provider is waiting for prior colon studies for comparison. C1 indicates a normal colon with routine screening to be continued. There are no visible abnormalities of the colon, no polyp ≥ 6 mm, or non-neoplastic findings such as lipoma or diverticula. C2 indicates indeterminate findings including 1-2 polyps 6-9 mm, or that a lesion ≥ 6 mm cannot be excluded, and recommends a shorter follow-up interval. C3 indicates detection of a polyp ≥ 10 mm, or 3 or more polyps 6-9 mm, and recommends concurrent colonoscopic follow-up. Finally, C4 indicates a colonic mass, most likely malignant, that "invades colonic lumen, demonstrates extracolonic invasion," for which surgical consultation is recommended. |
The standards assume that "well-performed" virtual colonoscopy is the diagnostic equivalent of conventional colonoscopy. However, there will be patients who insist on polypectomy when a lesion of any size is found; flexibility will be needed to manage these patients adequately, Zalis said.
Mammography established the precedent of surveillance, rather than resection, of lesions with less than a 2% likelihood of representing invasive malignancy, he said; the standard is applicable to most subcentimeter lesions detected at virtual colonoscopy.
Colonoscopy is recommended when three or more lesions 6-9-mm are detected in VC. Studies have demonstrated a higher number of synchronous lesions in this size category equates to a greater chance of developing advanced adenomas.
Lesions 1 cm or larger should be referred for colonoscopy. Larger lesions have an increased risk of harboring high-grade dysplasia or malignancy, and should therefore be removed.
Screening intervals of seven to 10 years are suggested for normal, negative VC exams. A range of reported performance statistics for VC studies indicates that seven to 10 years, compared to 10 years for conventional colonoscopy, represents a cautious screening interval for virtual colonoscopy, Zalis said. Still, cost-effectiveness criteria demand as long a screening interval as possible, so this standard is certain to evolve, he said.
As for diagnostic confidence, "If you're uncertain, bring the patient back for a shorter (surveillance) interval," Zalis said. "There is emerging data to suggest that we may want to include confidence scores; certainly academic centers may want to include a confidence score, and as that data emerges it may be more formally incorporated into the reporting standard."
Beyond the colon
Extracolonic findings should be evaluated judiciously to balance the well-being of the patient with the need to avoid unnecessary workup and its attendant risks, costs, and patient anxiety. To that end, VC exams are categorized into four categories, ranging from a nondiagnostic exam to serious extracolonic abnormalities requiring intervention.
| Table 3 covers the reporting of extracolonic findings. 0 represents a limited exam compromised by artifact, in which evaluation of extracolonic soft tissues is severely limited. E1 represents a normal exam or anatomic variant in which no extracolonic abnormalities are visible, or a normal variant such as retroaortic left renal vein. E3 indicates a clinically insignificant finding not requiring workup. These include, without limitation, simple cysts of the liver or kidney, cholelithiasis without cholecystitis, or hemangioma. E3 represents an exam with a likely insignificant finding, incompletely characterized. These are subject to local practice and patient preference, and workup may be indicated. The indications include, for example, minimally complex or homogeneously hyperdense cysts of the kidney. E4 indicates a potentially significant finding to be reported according to the "ACR Practice Guideline for Communication: Diagnostic Radiology." Examples include a solid renal mass, lymphadenopathy, aortic aneurysm, or a nonuniformly calcified parenchymal lung nodule ≥ 10 mm. |
"C-RADS reflects the currently available data," Zalis said. "It's certainly expected as our experience grows, these guidelines are going to evolve."
"The growth of (virtual colonoscopy) is great, but it requires us to organize the performance of reporting," he concluded. "We've developed C-RADS in an attempt to make a practical reporting scheme. It reflects radiology's role in the interpretation and collaborative management of colorectal carcinoma screening."
By Eric Barnes
AuntMinnie.com staff writer
November 2, 2004
Related Reading
Gastroenterology warning: Prepare for VC or regret it, October 6, 2004
VC faces off (again) against second-look colonoscopy, October 4, 2004
VC fares better as its own gold standard, September 8, 2004
What virtual colonoscopy misses might not matter, August 18, 2004
Copyright © 2004 AuntMinnie.com