Researchers at Stanford University School of Medicine in Stanford, CA, are developing a new molecular imaging system and technique designed to illuminate tumors and acquire data on more than two dozen tumor-related functions simultaneously.
The group is exploring Raman spectroscopy, which also has shown the ability to image functions of approximately one-trillionth of a meter in size. If proved safe and beneficial, Raman spectroscopy could provide information unavailable with current molecular imaging modalities.
The challenge with molecular imaging technologies that image cellular and molecular function, such as PET and SPECT, is that they are unable to image molecular events in multiple ways.
"For example, if you do a PET scan with one imaging agent, like FDG, if you want to image another process in the body, you have to wait for the FDG to decay and then bring the patient back and do a second scan," said Dr. Sanjiv Sam Gambhir, a professor of radiology and bioengineering and director of the molecular imaging program at Stanford. "If biology is going to move forward and imaging is to move with it, we have to be able to interrogate multiple events -- ideally, 10, 20, 30 events -- all simultaneously."
Details of the research were published in an advanced online issue of the Proceedings of the National Academy of Sciences (March 31, 2008).
Raman spectroscopy's roots date back to 1930, when Sir Chandrasekhara Venkata Raman received the Nobel Prize in physics for his work and discovery on the effect of scattering light. Until now, the Raman imaging technique has been used in industry for characterizing materials. "This is the first time the fundamental physical phenomenon known as Raman scattering has been used to create images of molecular processes in a living subject," Gambhir said.
New technology
Gambhir and fellow Stanford researchers began to explore Raman spectroscopy about three years ago, and have since developed an imaging device. As Gambhir said, "It isn't like you can buy an off-the-shelf Raman device."
The group also has developed nanoparticles, which act as imaging agents, to highlight various tumor functions. The nanoparticle becomes "excited by light and scatters the light to produce a Raman signature," Gambhir explained.
Conventional imaging agents, such as FDG or an MRI contrast agent, he added, are unable to produce a Raman signal.
The Raman spectroscopy device used for this imaging technique consists of lasers that emit the light that goes into an animal to excite the nanoparticles. Detectors based on CCD camera technology then detect the light returning from the nanoparticles that are attached to cancer cells. The size of the device is about the same as a conventional ultrasound system.
"Basically, it is like a microscope, but one adapted with lasers to provide high-energy light into the living subject, and then specialized detectors to detect the light coming out," Gambhir said.
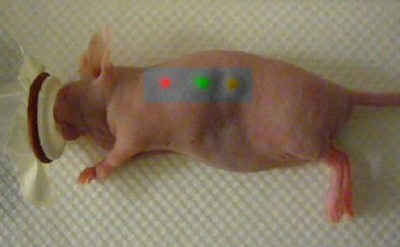
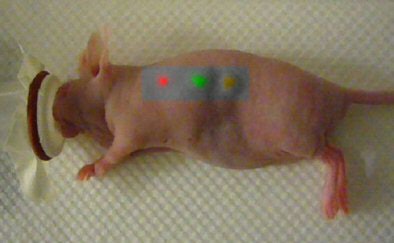 |
| This mouse was imaged with Raman spectroscopy after injection with two types of gold nanoparticles, each capable of producing a unique Raman signature in two separate locations. The third location is where an equal mixture of the two types of nanoparticles was injected into the same location. Laser light excitation leads to light scattering from these Raman nanoparticles. The inelastically scattered light is measured to create an image. The signal is directly related to the number of Raman nanoparticles at the given location. Image courtesy of Dr. Sanjiv Sam Gambhir and Stanford University School of Medicine. |
The group's goals now are to test the nanoparticles' capacities to gather multiple tumor functions. So far, cancer has been the focus of the group's efforts, with as many as five targets imaged at the same time. The targets include one present on new blood vessels associated with tumors.
Side effects
So far there have been no adverse side effects in testing on mice. The group has tested for toxicity by injecting carbon nanotubes over the course of five months and monitoring the animals' weight, blood pressure, and other reactions. "We have not seen any safety toxicity issues," Gambhir said, "but more studies need to be done."
When that stage is completed, researchers plan to apply to the U.S. Food and Drug Administration for clearance to use Raman nanoparticles on human patients, initially for the placement of the nanoparticles in the bowel to help detect colorectal cancer. "We are trying to get into human imaging in 18 to 24 months," he said.
With an endoscope in the bowel, as in a colonoscopy, "the tumor would light up and allow a gastroenterologist to see cancer in an application to detect early signs of colorectal cancer," Gambhir said. He sees the colorectal application as an appropriate first application for the technique, since the nanoparticles would be evacuated more easily from the bowel than if they were injected into a patient's bloodstream.
Gambhir expects Raman spectroscopy to be used for regional applications, such as breast imaging, colonoscopy, skin imaging for melanoma, and catheter-based imaging in the heart, esophagus, and trachea. Potential targets include "anything which is relatively close, say a few centimeters, from the surface of the skin," he said. "Imaging inside the brain, because of the skull, is not suited for this technology."
By Wayne Forrest
AuntMinnie.com staff writer
April 10, 2008
Related Reading
Good vibrations stave off blood-borne diseases, February 11, 2008
Raman spec reveals bone strength, October 28, 2005
Copyright © 2008 AuntMinnie.com




















