New evidence supports the use of SPECT/CT scans combined with serum PSA levels over PET/CT scans for evaluating early patient responses to Pluvicto treatment, researchers have reported.
The finding is from an international group led by researchers in Germany who compared pairs of baseline and interim SPECT/CT and PET/CT scans in 105 patients with metastatic castration-resistant prostate cancer (mCRPC ) who received at least two treatment cycles, noted lead author Lena Unterrainer, MD, of University Hospital LMU Munich in Germany. The study results were published April 24 in the Journal of Nuclear Medicine.
“These findings provide sufficient evidence to support the use of SPECT + PSA in daily routine over PET imaging without compromising quality of patient care,” the group wrote.
Lutetium-177 (Lu-177) prostate-specific membrane antigen (PSMA) radioligand therapy (Pluvicto, Novartis) was approved in the U.S. for treating patients with advanced prostate cancer in March 2022.
While the prognostic roles of post-therapeutic PET/CT and SPECT/CT imaging has been established in patients receiving the treatment, the comparative accuracy of the approaches for monitoring patients has not previously been investigated, the group wrote.
To that end, the researchers performed a retrospective analysis of the two techniques. The primary outcome was the prognostic value of post-therapeutic SPECT/CT versus PET/CT by RECIP 1.0 for overall survival. RECIP 1.0 is an emerging standardized framework for monitoring therapeutic efficacy of the treatment in early and advanced prostate cancer.
Additionally, the group tested the added value of a composite response classification method that combined PSA measurements and PET/CT and SPECT/CT responses by RECIP 1.0 (PSA + RECIP), given that serum PSA levels are typically used in clinical practice for treatment monitoring.
The researchers analyzed imaging from 105 men (median age, 75 years old) who underwent therapy between April 2019 and November 2023. Patients received a median of four treatment cycles. The median overall survival of patients was 12.3 months, and at the time of the study’s data cutoff, 77 patients (73%) had died.
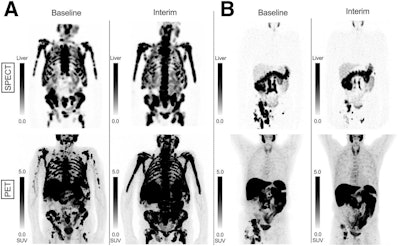 Patient examples of SPECT/CT and PET/CT RECIP 1.0. (A) SPECT and PET maximum-intensity projection (MIP) images in 74-year-old man with mCRPC. Serum PSA level at baseline was 449 ng/mL and slightly increased to 525 ng/mL after two cycles of Lu-177 PSMA. Eastern Cooperative Oncology Group (ECOG) score was 2 at baseline and increased to 3 after 2 cycles. The patient was classified by readers as RECIP-progressive disease on both SPECT and PET. Overall survival was 4.7 months. Two cycles of Lu-177 PSMA were administered. (B) SPECT and PET MIP images in 74-year-old man with mCRPC. Serum PSA level was 231 ng/mL at baseline and decreased to 12.5 ng/mL after 2 cycles of Lu-177 PSMA. ECOG score was less than 2 at baseline and after 2 cycles of Lu-177 PSMA. The patient was classified by readers as RECIP-partial response on both SPECT and PET. Overall survival was 14.3 months. Four cycles of Lu-177 PSMA were administered.Journal of Nuclear Medicine
Patient examples of SPECT/CT and PET/CT RECIP 1.0. (A) SPECT and PET maximum-intensity projection (MIP) images in 74-year-old man with mCRPC. Serum PSA level at baseline was 449 ng/mL and slightly increased to 525 ng/mL after two cycles of Lu-177 PSMA. Eastern Cooperative Oncology Group (ECOG) score was 2 at baseline and increased to 3 after 2 cycles. The patient was classified by readers as RECIP-progressive disease on both SPECT and PET. Overall survival was 4.7 months. Two cycles of Lu-177 PSMA were administered. (B) SPECT and PET MIP images in 74-year-old man with mCRPC. Serum PSA level was 231 ng/mL at baseline and decreased to 12.5 ng/mL after 2 cycles of Lu-177 PSMA. ECOG score was less than 2 at baseline and after 2 cycles of Lu-177 PSMA. The patient was classified by readers as RECIP-partial response on both SPECT and PET. Overall survival was 14.3 months. Four cycles of Lu-177 PSMA were administered.Journal of Nuclear Medicine
According to the findings, progressive disease determined by baseline SPECT/CT was associated with shorter overall survival compared with stable disease (hazard ratio [HR], 2.5) and with partial response (HR, 6.5).
Of 73 patients who underwent PET/CT and SPECT/CT after two cycles of treatment, seven (10%) had tumor progression shown by SPECT/CT, 30 (41%) by PET/CT, 22 (30%) by SPECT/CT plus PSA, and 30 (41%) by PET/CT plus PSMA.
“Our study is the first to provide a head-to-head comparison of SPECT/CT versus PET/CT for response evaluation during [Lu-177 PSMA-617] therapy,” the researchers wrote.
Ultimately, the study’s main finding is that post-therapeutic SPECT/CT by RECIP 1.0 after two cycles of treatment was prognostic for overall survival and can be used for evaluating treatment response and that SPECT plus PSA did not significantly differ from interim PET/CT, the group wrote.
Advantages of SPECT/CT over PET/CT include lower cost, wider availability, and less radiation, the researchers added.
“Our results can assist clinicians in making informed decisions on how to monitor the therapeutic efficacy of Lu-177 PSMA using PSMA-targeted imaging,” the researchers concluded.
The full study can be found here.





















