(Booth 6250) At this year's RSNA meeting, iCAD of Nashua, NH, will demonstrate the company's progress in expanding from its core emphasis on mammography computer-aided detection (CAD) into areas such as CT and MRI image analysis.
The company purchased MRI CAD developer CAD Sciences in July 2008; CAD Sciences had been developing products for therapy monitoring, identification of vascular diseases using contrast-enhanced MR angiography, and cancer detection using contrast-enhanced CT. Since then, iCAD has been adapting CAD Sciences' All Time Point (ATP) CAD analysis algorithms for use in prostate and breast MRI applications.
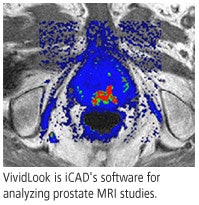
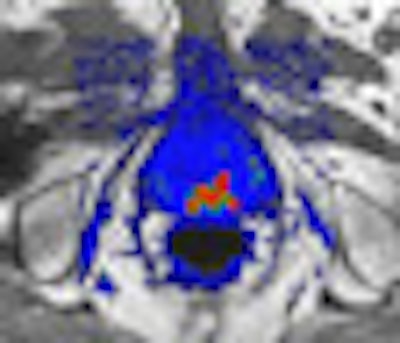
iCAD has used ATP technology to develop two different CAD servers, differentiated by clinical application. SpectraLook is designed for analyzing breast MRI studies, while VividLook performs the same function for prostate MRI. Both products have U.S. Food and Drug Administration (FDA) approval.
Both SpectraLook and VividLook export CAD analysis to CADvue, the company's image review and analysis workstation. CADvue enables clinicians to visualize the rapid enhancement and wash in and wash out of contrast, with images colorized based on pharmacokinetic curves. CADvue also supports report generation.
Also look for iCAD to discuss Colon CAD, an investigational application for identifying colon polyps in medical images. iCAD is working with the American College of Radiology's Image Metrix program on a clinical study designed to evaluate the impact of Colon CAD on the accuracy of interpreting CT colonography exams, according to the company. The software has not yet been approved by the U.S. Food and Drug Administration.
In iCAD's bread-and-butter mammography CAD market, you can expect the company to showcase its flagship SecondLook software, which works with both computed radiography (CR) and flat-panel mammography systems. iCAD will tout its receipt of FDA approval for use of SecondLook with the FCRm digital mammography system from Fujifilm Medical Systems USA of Stamford, CT.
Finally, iCAD will highlight TotalLook MammoAdvantage (TLMA), the company's second-generation digitizer for mammography images. The firm believes that TLMA offers improved image quality, enhanced image customization options, and workflow efficiency benefits to centers moving from analog to digital mammography.



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)




.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)