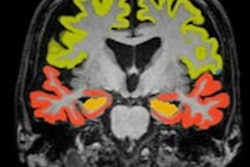
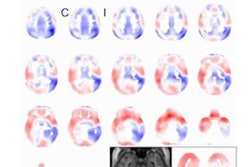
Artificial intelligence (AI) software developer Icometrix has received U.S. Food and Drug Administration (FDA) 510(k) clearance for the company's icobrain ep software for epilepsy detection on MRI.
Icometrix's icobrain ep software uses AI algorithms to facilitate the detection of subtle cortical abnormalities on the brain MR images of epilepsy patients. The software provides a quantitative report on brain asymmetry that indicates whether a patient has intractable -- or uncontrollable -- epilepsy, which is overlooked in as much as 45% of cases examined using traditional image evaluation, the company said.