Using the 2023 update of the American Association for the Study of Liver Diseases (AASLD) improves liver cancer surveillance via ultrasound, suggest findings published January 20 in Radiology.
Researchers led by Mei-Qing Cheng from the First Affiliated Hospital of Sun Yat-Sen University in Guangzhou, China, reported improved sensitivity and negative predictive value (NPV) with AASLD version 2023 over Ultrasound LI-RADS version 2017 and AASLD version 2018.
“These findings support the clinical implementation of this approach to enhance early HCC detection,” Cheng and co-authors wrote.
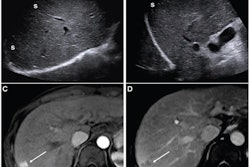
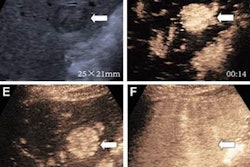
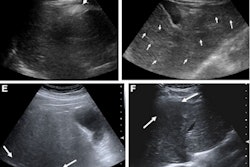
AASLD version 2023 incorporates new surveillance triggers, including ultrasound visualization score (VIS-C), increasing alpha-fetoprotein (AFP) level, and lesion growth. This guidance can improve sensitivity but at the cost of decreased specificity and higher recall rates. However, the researchers suggested these tradeoffs are justified by detecting more early-stage hepatocellular carcinomas (HCC) that can be more easily treated.
Cheng and colleagues assessed the surveillance performance of AASLD version 2023 for HCC detection. They compared this performance to that of LI-RADS version 2017 and AASLD version 2018 in people at high risk of HCC.
The prospective, multi-institutional study included 953 participants with a median age of 51 years, of whom 50 had HCC.
The new surveillance triggers for AASLD version 2023 achieved sensitivity values ranging from 8% (n = 4/50) to 48% (n = 24/50), with high specificity values of 94% (n = 849/903) to 99.4% (n = 898/903).
As an integrated algorithm, AASLD version 2023 achieved high sensitivity and NPV, superior to those of LI-RADS and AASLD version 2018. However, specificity did not improve from the previous version of AASLD and was lower than LI-RADS.
Comparison between AASLD version 2023, LI-RADS, AASLD version 2018 for HCC surveillance | |||
Measure | LI-RADS | AASLD version 2018 | AASLD version 2023 |
Sensitivity | 60% | 76% | 94% |
Specificity | 90% | 84% | 84% |
NPV | 98% | 98.5% | 99.6% |
| *All results achieved statistical significance. | |||
For early HCC, sensitivity for AASLD version 2023 remained superior compared with other algorithms (p < 0.001, p = 0.04).
On multivariable analysis, an AFP level of less than 20 ng/mL (odds ratio, 11.76; p < 0.001) and the absence of cirrhosis (odds ratio, 2.45; p = 0.03) were independently tied to false-positive findings for AASLD version 2023.
The study authors highlighted that the high sensitivity of AASLD version 2023 led to the false-negative rate being reduced to 6.3%.
“These findings emphasize the value of integrating imaging quality and longitudinal biomarkers into surveillance strategies rather than relying solely on lesion size or static AFP thresholds,” they wrote.
The team called for future studies to validate the performance of AASLD version 2023 across diverse populations with different races, liver disease etiologies, and body mass index categories.
Read the full study here.