Respiratory Bronchiolitis:
View cases of respiratory bronchiolitis
Clinical:
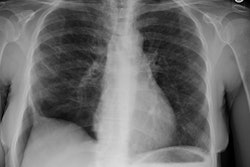
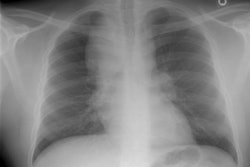
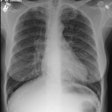
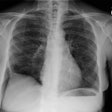
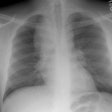
Respiratory bronchiolitis (RB) is a disorder seen in heavy cigarette smokers, and people with exposure to industrial/ environmental pollutants and probably represents a non-specific cellular reaction to inhaled irritants. RB is the most common form of smoking related lung injury and is usually asymptomatic [13]. Pathologically the changes of respiratory bronchiolitis consist of chronic bronchitis and Periodic acid-Schiff (PAS) positive brown macrophages are found within respiratory bronchioles and the adjacent alveoli. This finding seems to be specific for cigarette smoking. Respiratory bronchiolitis is usually mild and the majority of patients are asymptomatic [3,4,8].CXR: CXR can be normal or show non-specific, lower lobe, irregular small opacities. The disorder typically involves the lung in a patchy fashion.
Computed tomography: The majority of patients with respiratory bronchiolitis have normal CT scans. When abnormalities are present they consist of multiple areas of ground glass opacity most prominent in the upper lobes [2,4] and ill-defined centrilobular parenchymal and subpleural nodules within the mid to upper lung zones (similar to hypersensitivity pneumonitis, but nodules are less diffuse) [4,9] and bronchial dilatation.
Respiratory Bronchiolitis Associated Interstitial Lung Disease:
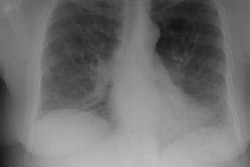
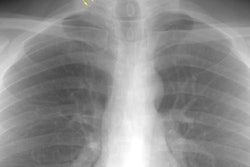
Respiratory bronchiolitis associated interstitial lung disease (RB-ILD) is the term given to the minority of smokers who become symptomatic secondary to respiratory bronchiolitis [12,13]. RB-ILD is a form of interstitial pneumonia in which respiratory bronchiolitis is associated with limited, peribronchiolar interstitial inflammation. Histolgically, the condition is identical to DIP with the additional criterion that it be most severe in the centrilobular regions of the secondary pulmonary lobule [7]. Because of the significant overlap in the clinical, imaging, and histologic features between RB-ILD and DIP the two disorders are considered to represent a continuum of the same process [10]. Affected patients are typically young (30-40 years old), heavy smokers (more than 30 pack years) with progressive mild dyspnea and variable productive cough [8]. Inspiratory crackles are present in one-half of patients and digital clubbing is rare [11]. The male-to-female ratio is reported to be 2:1.Pulmonary function testing is usually abnormal (in over 80% of patients) [14]. Patients typically have mixed restrictive and obstructive pulmonary function abnormalities and a reduction in DLCO [14]. Bronchoalveolar lavage fluid contains alveolar macrophages with golden, brown, or black pigment inclusions- findings which are indistinguishable from those seen in non-affected smokers. Treatment is to stop smoking and steroid therapy may also be used (however, some authors feel steroids have little role in the treatment of RB-ILD [11]). Prognosis is excellent with no evidence of progression to end stage fibrosis [11].
CXR: On CXR diffuse or basilar, bilateral, small linear and nodular opacities are found in 70% of cases, but the film can be normal in up to 21% of cases. Thickening of the walls of the central or peripheral bronchi can be seen in about 75% of cases [5]. Areas of atelectasis are found in just over 10% of cases. Adenopathy and pleural effusion are not seen.
Computed tomography: The CT features of RB-ILD overlap
with those of DIP, hypersensitivity penumonitis, and non-specific
interstitial pneumonitis [5]. On CT scattered areas of ground
glass opacification is the most common finding (66%) and can mimic
desquamative interstitial pneumonitis or respiratory bronchiolitis
[4]. Ill-defined centrilobular nodules (upper lobe predominant)
are also very common due to bronchiolar inflammation [3,10]. The
changes tend to have an upper lobe predominance, but may be
diffuse [3]. Septal thickening and irregular linear opacities are
infrequent [1]. Thickening of the walls of the central or
peripheral bronchi can be seen [5]. Scattered lobules of reduced
attenuation related to air trapping at expiratory imaging can also
be seen [13].
REFERENCES:
(1) RadioGraphics 1996;16:1009-1033
(2) J Thorac Imaging 1995;10(4):236-254 (p.251-252)
(3) Radiol Clin North Am 1998; Worthy SA, et al. Small airway diseases. 36 (1): 163-173
(4) AJR 1999; Heyneman LE, et al. Respiratory bronchiolitis, respiratory bronchiolitis associated interstitial lung disease, and desquamative interstitial pneumonia: Different entities or part of the spectrum of the same disease process? 173: 1617-1622
(5) Society of Thoracic Radiology Course Syllabus 2000; Lynch DA, et al. International concensus classification of idiopathic intestitial pneumonias. 52-56
(6) Radiographics 2003; Wittram C, et al. CT-histologic correlation of the ATS/ERS 2002 classification of idiopathic interstitial pneumonias. 23: 1057-1071
(7) AJR 2005; Miller WT, Shah RM. Isolated diffuse ground-glass opacity in thoracic CT: causes and clinical presentations. 184: 613-622
(8) Radiology 2005; Lynch DA, et al. Idiopathic interstitial pneumonias: CT features. 236: 10-21
(9) AJR 2005; Pipavath SJ, et al. Radiologic and pathologic features of bronchiolitis. 185: 354-363
(10) Radiographics 2007; Mueller-Mang C, et al. What every radiologist should know about interstitial pneumonias. 27: 595-615
(11) Radiographics 2008; Attili AK, et al. Smoking-related
interstitial lung disease: radiologic-clinical-pathologic
correlation. 28: 1383-1398
(12) Radiology 2013; Jin GJ, et al. Interstitial lung
abnormalities in a CT lung cancer screening population: prevalence
and progression rate. 268: 563-571
(13) Radiographics 2015; Sverzellati N, et al. American thoracic
society-European respiratory society classification of the
idiopathic interstitial pneumonias: advances in knowledge since
2002. 35: 1849-1872
(14) AJR 2018; Chung JH, et al. Screening for lung cancer:
incidental pulmonary parenchymal findings. 210: 503-513