accu
Amyloidosis:
View cases of amyloid
Clinical:
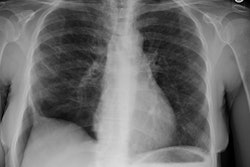
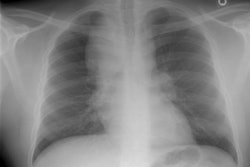
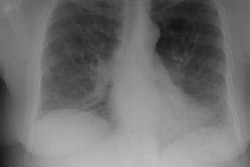
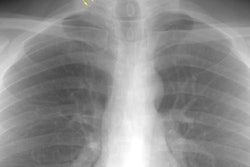
Amyloid is derived from the misfolding of proteins into long, unbranched fibrils of approximately 10 nm in diameter and several micrometers in length with a specific beta-sheet structure that is extremely stable and resistant to degradation by cells [21]. Amyloidosis is manifested by the extracellular deposition of that insoluble protein which characteristically stains with Congo red and produces a green birefringence when viewed under a polarizing microscope [7].The disorder can be localized (10-20% of cases) in which there are focal discrete depositions of amyloid, or systemic (80-90% of cases). Systemic disease can be classified as either primary, secondary, or familial. Primary and secondary disease account for the overwhelming majority of cases [7]. There is also a senile form of amyloidosis which typically involves the heart and brain [5] and about 10% of patients over the age of 80 will demonstrate pulmonary disease [4]. Typically senile amyloidosis is found incidentally at autopsy [4].In primary disease (amyloid light chain now referred to as AL disease) there is abnormal deposition of protein fibrils - usually a monoclonal immunoglobulin in the serum or urine (amyloid light chain (AL), typically a lamda or kappa light chain, a protein derived from the abnormal breakdown of immunoglobulin light chains associated with plasma cell dyscrasia [20]), but the spike is much smaller than that associated with muliple myeloma and radiolytic bone lesions are not found. At least 30% of patients with primary amyloidosis will eventually progress to multiple myeloma [7]. AL disease is the most common form of systemic amyloidosis in the US [26].Secondary amyloidosis (now referred to as amyloid A or AA amyloidosis) occurs when abnormally folded AA fibular protein (an acute phase reactant produced by the liver) accumulates in tissues [20]. AA amyloid is associated with chronic inflammatory disease such as rheumatoid arthritis, Reiters syndrome, Sjogrens syndrome, Crohn's disease, tuberculosis, Mediterranean fever, and parasitic infections [7,26].There are several other clinically relevant types of amyloid [26]. Transthyretin amyloid (ATTR) is a significant cause of morbidity and mortality related to cardiomyopathy [26]. Beta-amyloid deposition in the brain is associated with Alzheimer disease and cerebral amyloid angiopathy is associated with cerebral hemorrhage [26].In systemic disease the respiratory system is involved in 50% of cases and involvement is usually characterized by a diffuse interstitial pattern. Various forms of pulmonary disease can be seen, including: diffuse parenchymal disease, nodular parenchymal disease, and tracheobronchial involvement. Isolated mediastinal nodal involvement/mediastinal amyloid masses are very rare in the absence of pulmonary involvement, but have been reported [6]. Calcification can be seen in up to 50% of such cases [6].
1- Diffuse alveolar septal pulmonary disease:
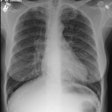
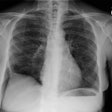
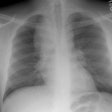
Diffuse alveolar septal pulmonary involvement is nearly always associated with multi-organ system involvement (ie: systemic disease). In these cases amyloid is deposited between the capillary lumen and the alveolar lining cells, leading to progressive dyspnea, pulmonary restriction, and diminished diffusing capcity [5]. The disorder can be characterized by the presence of interlobular septal thickening (particularly in the bases), multiple pulmonary micronodules, patchy areas of ground glass attenuation, and less commonly areas of basal-predominant subpleural consolidation. Honey-combing has also been described in these patients. Hilar and mediastinal adenopathy is common. Patients with systemic amyloid and pulmonary involvement frequently experience dyspnea and have a poor long term prognosis with a median survival of 16 months. [1,4] Despite their poor prognosis, the pulmonary is disease is responsible for the patients death in only about 10% of cases [4].Pulmonary light chain deposition disease is a pathologic distinct entity with similar diffuse and nodular patterns [26]. The deposits do not stain with Congo red [26]. CT images show multiple thin-walled cysts, often with vessels traversing the cysts, and pulmonary nodules without a specific zonal distribution [26].
2- Nodular pulmonary disease:
Nodular amyloid lesions (amyloidomas) are uncommon and are usually NOT associated with extra-pulmonary amyloid (systemic disease). The disorder rarely produces any clinical pulmonary impairment and patients are typically asymptomatic. There are solitary or multiple well-defined, peripheral, 1-3 cm masses which may cavitate. Uncommonly, the lesions can have ill-defined or spiculated margins and can mimic a malignancy. The nodules are usually concentrated in the lower lobes [13]. Calcification can be found in 20-50% of nodules by CT. The nodules typically display slow growth over years. Hilar adenopathy may be seen. [1,4] In rare instances, the condition may be associated with cysts most commonly in patients with Sjogren syndrome [13]. The condition can also be seen in association with MALT lymphoma [26]. Biopsy of the lesions most often show localized AL amyloid and 18F-florbetapir imaging has been used to confirm pulmonary amyloid deposits [26].
3- Tracheobronchial disease:
Tracheobronchial disease is found in 23-53% of patients with localized pulmonary amyloidosis (mean age at presentation is 62 years) (4). It most commonly causes diffuse/segmental or focal narrowing of the trachea or bronchi by multifocal nodular submucosal deposits that protrude into the airway. In about 15% of patients it appears as a focal endobronchial mass (amyloidoma). Tracheobronchial disease will also involve the posterior membranous wall of the trachea, unlike other conditions such as relapsing polychondritis and tracheobronchopathia osteochondroplastica [26]. The overlying mucosa is usually intact. Calcification is uncommon (fewer than 10% of cases). Affected patients are usually asymptomatic, but may complain of dyspnea, hemoptysis, or recurrent infection. For obstructing endobronchial lesions, recurrent bronchoscopic resection is usually performed. Laser therapy has also been used. On CT, the tracheal wall is markedly thickened- affecting both the anterior cartilagenous and the posterior membranous walls. [2,3,4]
Cardiac amyloid:
Cardiac amyloidosis is caused by the myocardial accumulation of misfolded protein deposits (amyloid fibrils) [29]. Cardiac involvement usually affects patients over the age of 65 years [11,22]. The deposition of insoluable amyloid proteins in the myocardial interstitium results in diffuse myocardial thickening, impaired contractility, restrictive physiologic features , progressive diastolic and systolic dysfunction, CHF, conduction disturbances with sudden death, and occasional ischemia [11,22]. Cardiac involvement is the primary cause of death in approximately half of patients with primary amyloidosis [9].
The major types of cardiac amyloidosis accounting for 95% of cases [21] are cardiac amyloid light-chain (AL) and two forms of transthyrethin-related cardiac amyloid (ATTR)- which is a hereditary mutant form (ATTRm) and a non-mutant wild-type (ATTRwt) [14,19,23]. Cardiac involvement is rare in patients with AA amyloidosis (occurs in only 2% of patients) [11,16].
In the AL subtype, the fibril proteins are composed of immunoglobulin light chains that are produced by abnormal monoclonal plasma cells in the bone marrow [14,22]. The underlying diseases associated with AL include multiple myeloma, primary macroglobulinemia, and monoclonal gammopathy of uncertain significance [22]. AL is a systemic disease that affects the kidneys and other organs, but prognosis is largely driven by the infiltrative cardiomyopathy [21,31]. In AL, myocardial deposition of amyloid induces cardiomyocyte necrosis and interstitial fibrosis [22]. Cardiac involvement occurs in 50-90% of patients with systemic AL amyloidosis (AL amyloid can occur in patients with plasma cell disorders such as myeloma, plasma cell malignancies, or Waldenstroms macroglobulinemia [18]) [11,16,24]. The AL subtype is the most common and associated with rapid progression and a poor prognosis - there is a greater than 50% mortality within 6 months of diagnosis if untreated [14] and a median survival of 12 months [15,20,23,24]. Other authors indicate there is a male predominance and most patients with AL cardiac amyloid often develop significant heart failure with poor survival beyond 2 years of symptoms, unless treated with heart transplant and concomitant bone marrow transplant [18]. Treatment for the AL subtype involves plasma cell directed chemotherapy with daratumumab in combination with cyclophosphamide, bortezomib, and dexamethasone [14,31]. Treatment with proteasome inhibitor therapy has also significantly improved survival outcomes [22].
The ATTR subtype can be due to an autosomal-dominant mutation (familial cardiac amyloidosis or mutant-type ATTRm) or a wild-type variant mutation (ATTRwt) in the TTR gene. The mutation causes destabilization of the transthyretin protein (TTR- which is normally a stable soluable tetramer) into misfolded monomers or dimers that form fibril sheets and get deposited into the heart and other organs [14]. TTR is normally a carrier of thyroxine and retinol bound to retinol-binding protein that is largely produced by the liver and, in smaller amounts, by the retinal epithelium and choroid plexus [21]. Depending on the type of mutation, patients with ATTR may have a predominant cardiac phenotype (V122I- valine to isoleucine amino acid substitution at position 122 mutation), a predominantly neuropathic pehnotype (early onset V30M- valine at amino acid 30 to methionine mutation), or a combination of the cardiac and neuropathic phenotype [32].
With the familial subtype, amyloid deposition is limited to the conduction system and the condition most frequently manifests as sinus node dysfunction [20]. Cardiac involvement is seen in 30-90% of patients with ATTRm amyloid (depending on the gene variant) and in almost all patients with ATTRwt amyloid [24].
ATTRwt is also referred to as senile systemic amyloidosis- a slowly progressive disorder with longer survival predominantly in men aged 65 or older (the male-to-female ratio is 20-50:1) [14,15,22]. ATTRwt is increasingly recognized as an important cause of heart failure with preserved ejection fraction in older adults [31]. In the wild-type variant myocardial, TTR infiltration is present in 25% of individuals older than 80 years [22]. Other authors indicate that ATTRwt can be found in 10-16% of older patients with heart failure [24]. ATTRwt patients tend to have less aggressive cardiac manifestations and can be affected by carpal tunnel syndrome in 40-60% of patients [22].
ATTRm (transthyretin cardiac amyloidosis) affects patients of a younger age and has mixed clinical presentations of peripheral or autonomic neuropathy in addition to cardiomyopathy (cardiac involvement is seen in about 25% of patients with ATTRm) [23,27]. Other authors indicate that the age of onset can be variable ranging from the 3rd to 8th decade [27]. Compared to patients with AL disease, the ATTR subtype has a more favorable prognosis- with ATTRm patients having a median survival of 10-20 years after onset (although cardiac involvement is associated with a worse prognosis).
ATTR cardiomyopathy is increasingly recognized as a cause of heart failure with preserved ejection fraction in older patients [32]. Untreated patients with ATTR cardiomyopathy and preserved ejection fraction have a poor survival of 2.5-3.5 years (other authors suggest the median survival is 4.8 years without treatment [31]) [32]. Those patients are also at increased risk fir developing heart failure or for needing to undergo transplant within a few years [32].
Treatment options for the ATTR subtype include orthotopic liver transplant (familial ATTR only) and agents designed to stabilize the TTR tetramer (diflunisal and tafamadis) or reducing/silencing TTR synthesis in the liver using RNA interference therapy (patisian, vutrisiran, inotersen, and eplontersen) [14,22]. With this treatment, small interfering RNA binds to conserved sequences on TTR messenger RNA, degrading the messenger RNA and reducing TTR gene expression [22]. Tafamadis (which is approved for therapy of ATTR) binds to TTR and inhibits tetramer dissociation and amyloidogenesis [24]. ATTRwt patients treated with tafamidis have a reduced all-cause mortality, decreased cardiovascular related hospitalizations, and slower declines in functional capacity and quality of life [22]. However, the agent is less effective in patients that have already developed advanced heart failure (class 3) [31]. Antisence oligonucleotide therapy can also reduce TTR translation by suppressing gene expression [22].
Diagnosis:
Endomyocardial biopsy is the reference standard for detecting cardiac amyloid, but the procedure is invasive and false-negative rates are high [22].
Cardiac imaging:
ECG demonstrates low voltage (
On echocardiography, cardiac amyloid most commonly produces symmetric left ventricular wall thickening [8], but involvement of all 4 chambers is common [11]. Cardiac amyloid may be indicated by the presence of a symmetric increase in biventricular wall thickness (LV wall thickness > 12mm and often > 15 mm) [22]. Wall thickening is typically more prominent in patients with ATTR as compared to AL disease [22]. Diastolic dysfunction may be the earliest detectable ECG abnormality as reflected by alterations in the trans-mitral Doppler early-filling velocity ratio [22]. When compared to hypertrophic cardiomyopathy, affected patients demonstrate inferior ventricular wall contractility [8]. Thickening of the right atrial free wall and intra-atrial septum (>6mm) can also be seen in the disorder (and these areas are not typically involved in hypertrophic cardiomyopathy) [8,12]. Thickening of the right ventricular free wall of more than 6 mm has been shown to be a specific marker in diagnosing cardiac amyloid [8]. Reduced myocardial longitudinal function (ie: strain) is characteristic of myocardial involvement and strongly associated with survival [22]. Typically, the pattern of myocardial longitudinal strain follows a regional gradient from the base to the apex; this pattern is described as apical sparing and differs from other causes of LV thickening [22].
Associated pericardial and pleural effusions can be seen in up to 50% of cases [8].
Nuclear medicine:
Radionuclide imaging with bone imaging compounds can be used for imaging cardiac amyloid (ATTR disease) [15,21]. However, a negative scan does not exclude AL disease due to the exams poor sensitivity (<50%) and specificity (<50%) [15,24]. Additionally, 10-30% of patients with AL cardiac amyloid can show cardiac uptake with bone-seeking tracers [32]. As such, it is important to also be aware of the patient's clinical data including serum or urine monoclonal protein and serum light chain levels [32].
Bone avid tracers bond to microcalcifications in the amyloid fibrils [24]. Using specific stains and immunohistochemistry, a greater density of microcalcification has been reported in ATTR patients and a greater density of macrophages in AL patients- this may explain a calcium-based binding mechanism for tracer uptake in ATTR [21].
Tc-99m-pyrophosphate, Tc-99m-DPD, and Tc-99m-HMDP are agents used to evaluate for the presence of cardiac amyloid [23]. Planar radionuclide imaging has demonstrated a sensitivity of 91-99% and a specificity of 86-92% for diagnosing ATTR in patients with suspected cardiac amyloid [25]. Imaging with Tc99m-medronate lacks sensitivity and should not be performed for the evaluation of cardiac amyloid [24]. 18F-NaF has also been found to be inferior to Tc-99m-pyrophosphate in the detection of cardiac ATTR amyloid [29].
For the three tracers used for imaging, between 10-20 mCi (370-740 MBq) is administered [32]. Imaging is performed using a large FOV camera with low-energy high resolution collimators [32]. Planar images of the chest are acquired using 1 256x256 matrix and at least 500,000 counts in the anterior, LAO, and left lateral projections [32]. Relying on planar images alone can result in ATTR misclassification in 2-28% of patients and the use of visual assessment of planar images and H/CC ratio alone are insufficient for the diagnosis of ATTR [32]. SPECT imaging is required to aid in differentiation between myocardial uptake and blood pool or extra cardiac activity [32]. SPECT imaging of the chest is acquired using 128x128 matrix, 60 steps, and 3 degree angular steps [32]. SPECT CT further aids in localization of tracer activity and is preferred [32]. For PYP scintigraphy, SPECT imaging should be performed 2 or 3 hours following tracer administration [32]. If excess blood pool activity is observed at 2 hour SPECT imaging, then another acquisition at 3 hours is recommended [32]. For DPD or HMDP SPECT imaging is performed at 2 or 3 hours following tracer administration [32].
Tc-99m-pyrophospate:
Guidelines recommend planar imaging at 1 or 3 hours (or both) after injection [21]. Imaging at one hour is associated with higher sensitivity, while 3 hour imaging is associated with higher specificity [22]. The pattern of myocardial uptake should be described as focal, diffuse, or both focal and diffuse [32].
Interpretation is based on a semiquantitative visual grading of tracer uptake- grade 0 = no myocardial uptake; grade 1 = mild uptake - myocardial < rib (myocardial uptake less than bone); grade 2 = moderate uptake - myocardial equal to ribs/bone uptake; and grade 3 =high uptake - myocardial > rib/bone uptake [21]. A visual score of 0 is not suggestive of TTR amyloidosis [29]. A score of 1 is considered equivocal, while grade 2 or 3 are considered positive [21,29].
A quantitative heart-to-contralateral chest ratio (H/CC) can also be used (mean ROI heart counts to mean contralateral identical chest ROI counts) [21,23,32]. For this method, a region of interest is drawn over the heart, which is divided by background counts in a copied and mirrored ROI over the contralateral chest [22]. Use of this method normalizes for spillover from the ribs [22]. Values greater than 1.5 being highly specific for ATTR (sensitivity 97% and specificity 100%) [21,23]. Other authors indicate that a H/CC ratio > 1.5 at one hour or >1.3 at 3 hours supports a diagnosis of ATTR, as long as SPECT imaging confirms myocardial uptake [32]. Additionally, a heart-to-contralateral ratio higher than 1.6 has been shown to independently predict a worse survival [22,32].
The principle confounder of planar imaging is the presence of tracer in the cardiac blood pool resulting in false elevated tracer activity and false positive exams [21]. SPECT imaging should be performed to distinguish between blood pool and myocardial activity and improve diagnostic accuracy [21,29,32]. Additionally, studies suggest no added advantage of 3 hour imaging if both planar and SPECT imaging are performed at 1 hour [21].
False positive exams can occur due to increased blood pool activity, chemotherapy or drug associated myocardial toxicity (hydroxychloroquine cardiotoxicity), recent myocardial infarction, extensive mitral annular calcification, and pericarditis [32]. False-negative exams may occur in early disease and in certain hereditary variants [32].
Increased chest wall uptake due to a rib fracture or assymmetric costochondral calcification can lead to over estimation of mean counts in either the heart ROI or contralateral ROI [32]. A large pleural or pericardial effusion may cause underestimation of the mean count in either ROI [32].
Myocardial scintigraphy using bone-seeking tracers is not currently recommended for monitoring treatment response [32].
99mTc-DPD (99mT-HMDP):
Imaging is performed 3 hours following tracer injection [24]. The agent can be used to distinguish ATTR and AL cardiac amyloid [23]. The agent may allow early diagnosis even before the appearance of ECG findings [23].
On planar imaging, myocardial retention is graded on a semiquantitative visual scoring system (Perugini score)- 0= no cardiac uptake; 1= mild cardiac uptake less than general bone/rib uptake; 2= moderate cardiac uptake equal to general bone/rib uptake; and 3= strong cardiac uptake with mild or absent bone/rib uptake [23,24]. Grade 0 is considered not suggestive of ATTR amyloid, grade 1 is equivocol, and grades 2 and 3 are considered strongly suggestive for cardiac ATTR amyloid [24].
On quantitative evaluation, on HDMP imaging activity in the lungs and soft tissues/muscle can result in an inaccurate H/CC ratio [32]. For DPD and HMDP imaging, a heart to whole body ratio (H/HB) should be calculated [32]. On the anterior projection, ROIs are placed over the heart, both kidneys, and the urinary bladder [32]. In addition, a rectangular ROI is placed around the whole body [32]. Total counts are recorded for each ROI, and total counts in the heart ROI are divided by the difference between total counts in the whole body ROI and kidney and urinary bladder ROI [32]. Consensus regarding cutoff values for the H/WB ratio are lacking [32].
Treatment with tafamidis can result in regression of myocardial 99mTc-DPD uptake [30].
PET imaging of cardiac amyloid:
Cardiac amyloid can be imaged using amyloid-binding tracers, such as 11C-PIB, 18F-florbetapir, 18F-florbetaben and 18F-flutemetamol have recently been studied to detect cardiac amyloid [17,18,19,28]. However, the agents cannot distinguish between ATTR and AL disease [21]. Preliminary studies show higher myocardial tracer uptake in AL and ATTR patients compared to controls and higher myocardial uptake in AL patients than in those with ATTR [21,22]. However, the clinical utility of PET tracers may be limited by their inability to differentiate AL from ATTR cardiac amyloid [32].
For 11C-PIB, a 10-20 minute static cardiac scan starting 10 minutes after injection of 185-370 MBq of the tracer is performed [31]. The exam is considered positive when the myocardial uptake of the agent is visually discernible from the blood pool [28]. Patients with 11C-PIB myocardial uptake have been shown to have a worse one year survival rate (45.5%) compared to those without tracer uptake (82%) [28].
18F-NaF has also been explored for diagnosing ATTR disease (supporting a calcium binding hypothesis) [21].
I123-MIBG: On I123-MIBG imaging, a reduced heart-to-mediastinum uptake ratio below 1.6 is associated with a poor prognosis in patients with ATTRm with a 5-year mortality of 42%, compared to only 7% for patients with an uptake ratio equal to or greater than 1.6 [22].
MRI:
Cardiac MRI has a reported sensitivity of 94% and a specificity of 80% for the diagnosis of cardiac amyloidosis [27]. Infiltration of the myocardium leads to increased myocardial mass and impaired ventricular relaxation (diastolic dysfunction) [27]. Overtime, systolic dysfunction develops and leads to a reduction in stroke volume and LV ejection fraction with a relative preservation of apical contractility [27]. Structural abnormalities include atrial wall thickness > 6 mm at the atrial septum or atrial free wall, biatrial dilatation, and ventricular wall thickening > 12mm at the septum [27].
LGE MRI is an established method for identifying cardiac amyloid with sensitivity and specificity rates approaching 85-90% [22]. The typical LGE pattern involves global subendocardial LGE distributed in a non-coronary artery territory, that can be patchy or transmural, and mainly in the basal segments [22,27]. The LGE pattern is more extensive and transmural with ATTR than with AL disease, and RV involvement is also common with ATTR [22]. The presence of transmural LGE in patients with CA indicates a poor prognosis, regardless of the amyloid type [22].
Cerebral amyloid angiopathy:
Cerebral amyloid angiopathy (CAA) is an important cause of primary intracranial hemorrhage in older patients [26]. The disease is characterized by deposition of beta-amyloid in the media and adventitia of small arteries within the brain and leptomeninges [26]. The incidence of CAA rises with age in patients older than 60 years, but can be seen in the 3rd-5th decades of life in hereditary disease such as Dutch-type hereditary CAA [26].
Features of CAA on CT or MR include multiple cerebral hemorrhages or cerebral microbleeds restricted to lobar, cortical, or cortical-subcortical regions, or alternatively a single hemorrhage or microbleed in these regions in the presence of cortical superficial siderosis [26]. Cortical superficial siderosis occurs owing to hemosiderin deposition in the setting of recurrent hemorrhage and is readily detected on susceptibility-weighted MR images as areas of low signal intensity lining the leptomeninges with associated blooming [26]. Susceptibility-weighted imaging is also valuable for the detection of cerebral microbleeds which appear as rounded hypointense dots that are not visible at CT or with conventional MR sequences [26].
REFERENCES:
(1) AJR 1997; Pickford HA, et al. Thoracic cross-sectional imaging of amyloidosis. Feb 168 (2):351-355
(2) J Thorac Imaging 1995; Shepard JA. The bronchi: an imaging perspective. 10 (4):236-254 (p.237)
(3) ACR Syllabus #40: p.267-269
(4) Ann Intern Med 1996; 124 (4): 407-413
(5) N Engl J Med 1996; Scully RE, et al. Case records of the Massachusetts general hospital. Weekly clinicopathological exercises. Case 23-1996. 335 (4): 266-273
(6) Thorax 1995; Hiller N, et al. 50(8): 908-909
(7) Radiographics 2004; Georgiades CS, et al. Amyloidosis: review and C manifestations. 24: 405-416
(8) AJR 2007; Hansen MW, Merchant N. MRI of hypertrophic cardiomyopathy: Part 2, differential diagnosis, risk stratification, and post treatment MRI appearances. 189: 1344-1352
(9) J Nucl Cardiol 2008; O'Hanlon R, et al. Evaluation of non-ischemic cardiomyopathy using cardiovascular magnetic resonance. 15: 400-416
(10) Radiographics 2009; Cummings KW, et al. A pattern-based approach to assessment of delayed enhancement in nonischemic cardiomyopathy at MR imaging. 29: 89-103
(11) Radiographics 2009; Sparrow PJ, et al. CT and MR imaging findings in patients with acquired heart disease at risk for sudden cardiac death. 29: 805-823
(12) Radiology 2012; O'Donnell DH, et al. Cardiac MR imaging of nonischemic cardiomyopathies: imaging protocols and spectra of appearances. 262: 403-422
(13) Radiology 2012; Lee AY, et al. Case 182: Pulmonary amyloidosis. 263: 929-932
(14) J Nucl Cardiol 2014; Aljaroudi WA, et al. Role of imaging in the diagnosis and management of patients with cardiac amyloidosis: state of the art review and focus on emerging nuclear techniques. 21: 271-283
(15) J Nucl Cardiol 2014; Dorbala S. Imaging cardiac amyloidosis: an opportunity for nuclear cardiology. 21: 1043-1044
(16) Radiographics 2015; Czeyda-Pommersheim F, et al. Amyloidosis: modern cross sectional imaging. 35: 1381-1392
(17) J Nucl Cardiol 2016; Kero T, et al. Accurate analysis and visualization of cardiac 11C-PIB uptake in amyloidosis with semiautomated software. 23: 741-750
(21) J Nucl Med 2020; Masri A, et al. Molecular imaging of cardiac amyloidosis. 61: 965-970
(27) Radiographics 2022; Fadl SA, et al. Cardiac MRI of hereditary cardiomyopathy. 42: 625-643