Eosinophilic granulomatosis with polyangiitis
Previously known as - Churg-Strauss Syndrome or Allergic Angiitis
View cases of Churg-Strauss
Clinical:
Churg-Strauss is a rare granulomatous vasculitis with no sex
predominance that primarily affects patients between the ages
40-50 years (mean age of onset is 38 years [14]). It is the least
common of the angiitis and granulomatous diseases of the lungs. It
is strongly associated with a history of longstanding progressive
severe asthma and marked peripheral eosinophilia is found in
30-40% of patients. Patients also frequently complain of allergic
rhinitis (55-70%), nasal polyps, and sinusitis [6].
The disorder has three distinct phases: 1- a prodromal phase that
may persist for many years consisting of asthma and often
preceding allergic rhinitis/rhinosinusitis; 2- a phase of marked
peripheral blood eosinophilia and eosinophilic tissue/organ
infiltrates; and 3- a life-threatening vasculitic phase [9,12].
However, the 3-phases do not always manifest in this order [9].
The American College of Rheumatology developed a set of criteria
for the diagnosis of Churg-Strauss syndrome. The six criteria are
1- asthma; 2- peripheral blood eosinophilia greater than 10% of
the WBC differential count; 3- mono- or polyneuropathy; 4-
migratory or transient pulmonary opacities; 5- paranasal sinus
abnormality/sinusitis; and 6- extravascular eosinophils at biopsy.
A patient with vasculitis is considered to have Churg-Strauss if
at least 4 of these 6 criteria are met [2,9,12]. These diagnostic
criteria have shown a sensitivity of 85% and a specificity near
100% [10].
The vasculitis associated with the disorder is a systemic process that affects both arteries (usually small arteries) and veins [7,8]. The lung is the most commonly involved organ with upper airway disease and pulmonary involvement in about 70% of patients [3]. However, pulmonary hemorrhage is much less common than in other small vessel vasculitides [9]. Cutaneous skin lesions (purpura or nodules) are the most common extra-pulmonary finding and occur in up to two-thirds of patients. A migratory small joint arthritis occurs in 50% of patients [6]. A peripheral mononeuritis multiplex occurs in up to 75% of patients [6]. Glomerulonephritis is uncommon, but renal involvement (renal vasculitis) can be seen in up to 38% of patients. Cardiac involvement is more common (13-47% of cases) than in Wegeners [5] and cardiac involvement is the main cause of morbidity and mortality [9,10]. Cardiac abnormalities include eosinophilic myocarditis and coronary vessel vasculitis [6]. Other symptoms include headaches, fever, malaise, arthralgia, and gastrointestinal symptoms. Abdominal pain is very common, usually secondary to an eosinophilic gastroenteritis or vasculitis [6]. Elevated serum levels of IgE are typical and rheumatoid factor levels are elevated in 52% of patients [5]. About 40-75% of patients with Churg-Strauss sydrome are ANCA positive and the p-ANCA staining pattern predominates [10]. Further evaluation with an ELISA test is usually performed to ensure that the p-ANCA staining pattern is the result of myeloperoxidase (MPO) antigens and not some other nuclear antigen [6].
Treatment with corticosteroids is generally effective- 5 year
survival approaches 85%, but relapse is common. The addition of
cyclophosphamide can result in a decreased relapse rate, but must
be weighed against the potential complications of this therapy
(15-45 fold increased risk for urological malignancies and
hemorrhagic cystitis) [6]. The asthma component of the disorder,
however, is rarely affected by therapy [11]. The reported 5-year
survival is 60-80% [13].
X-ray:
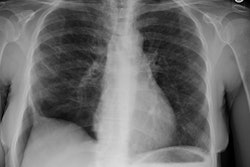
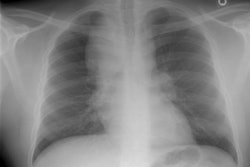
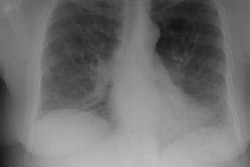
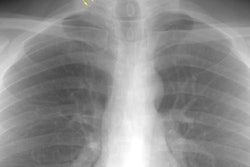
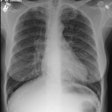
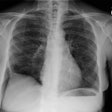
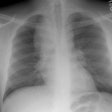
CXR: The most common finding on CXR is transient, bilateral, predominantly peripheral (50% of cases), nonsegmental areas of opacification/consolidation with predilection for any lung zone [9]. Pulmonary opacities can be found in 26-77% of cases and the film is normal in about 25% of patients. Cavitation is rare. Pulmonary infiltrates may be transient (similar to those seen in patients with Loffler's or chronic eosinophilic pneumonia) or nodular. Extensive air-space opacities in the setting of hemoptysis suggests massive intraalveolar hemorrhage. Pleural effusions are seen in 5-30% of cases and can be eosinophilic (other authors suggest pleural effusion can be seen in up to 50% of cases and may be secondary to an eosinophilic pleuritis [14]). Hilar nodal enlargement has occasionally been reported [3].
Computed tomography: Pulmonary parenchymal findings are
described as having a bronchocentric pattern [14] and include
peripheral areas of parenchymal consolidation/ground glass
attenuation similar to chronic eosinophilic pneumonia in up to 90%
of patients [9]. These usually have a symmetric distribution and
often a peripheral predominance [9], without a zonal preference
(10). Less commonly, the abnormalities have a bronchial or
patchy/random distribution [9]. A recent article suggested the
most common findings on CT to consist of small nodules (63%),
GGO's (53%), bronchial wall thickening (53%) or dilatation (53%),
consolidation (42%), interlobular septal thickening (42%) and
mosaic attenuation (47%) [10]. Cavitary lesions are uncommon (as
opposed to granulomatosis with polyangitis in which cavitation is
common) [14].
Other authors suggest less common findings include parenchymal
nodules (from 5 mm to 3.5 cm), which may be cavitary or contain
air bronchograms. Airway involvement produces bronchial dilatation
and bronchial wall thickening, small centrilobular nodules, and
tree-in-bud opacities [9]. A relatively common finding is the
presence of interlobular septal thickening (about 50% of patients)
[9].
Unilateral or bilateral pleural effusions are seen at CT in
10-50% of patients and may be related to cardiac dysfunction or
eosinophilic pleuritis [9]. Mediastinal adenopathy is seen in less
than 25% of patients [14]. Although it is not part of the
diagnostic criteria, cardiac involvement in EGPA is common
(prevalence 66% clinically and 92% at autopsy) [14].
REFERENCES:
(1) Radiol Clin North Am 1991; Sept 29 (5): 973-982
(3) J Comput Assist Tomogr 1997; 21 (6): 920-930
(4) ACR Syllabus #40: p.433-34.341-42
(5) Radiographics 1998; Frazier AA, et al. Pulmonary angiitis and granulomatosis: Radiologic-pathologic correlation. 18: 687-710
(6) Thorax 2000; Conron M, et al. Churg-Strauss syndrome. 55: 870-877 (No abstract available)
(7) Radiology 2002; Hansell DM, et al. Small-vessel diseases of the lung: CT-pathologic correlates. 225: 639-653
(8) AJR 2005; Marten K, et al. Pattern-based differential
diagnosis in pulmonary vasculitis using volumetric CT. 184:
720-733
(9) Radiographics 2010; Castaner E, et al. When to suspect pulmonary vasculitis: radiologic and clinical clues. 30: 33-53
(10) Radiology 2010; Chung MO, et al. Imaging of pulmonary
vasculitis. 255: 322-341
(11) AJR 2013; Nemec SF, et al. Noninfectious inflammatory lung
disease: imaging considerations and clues to differential
diagnosis. 201: 278-294
(12) Radiographics 2016; Katre RS, et al. Cardiopulmonary and
gastrointestinal manifestations of eosinophil-associated diseases
and idiopathic hypereosinophilic syndromes: multimodality imaging
approach. 36: 433-451
(13) AJR 2017; Bernheim A, McLoud T. A review of clinical and
imaging findings in eosinophilic lung diseases. 208: 1002-1010
(14) Radiographics 2020; Naeem M, et al. Noninfectious
granulomatous diseases of the chest. 40: 1003-1019