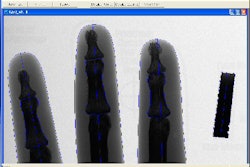
GE Healthcare has received U.S. Food and Drug Administration clearance for OEC Fluorostar, a digital C-arm designed to provide fluoroscopic images during diagnostic, surgical, and interventional procedures.
OEC Fluorostar features a compact design and a flat-panel display, with access to all functions available from either the left- or right-side touch-screen interface, according to the Waukesha, WI-based vendor. The system is capable of accommodating clinical applications such as cholangiography, endoscopy, urologic, orthopedic, neurologic, vascular, cardiac, and stone localization, as well as critical care and emergency room procedures, GE said.
In other announcements made by GE at the 2005 annual meeting of the American Academy of Orthopaedic Surgeons (AAOS) in Washington, DC, the firm is introducing NAV Axcess Needle, a surgical navigation instrument that allows surgeons to track their hand instruments as a virtual tool image superimposed over x-ray images.
In customer news, GE said it has signed an agreement to supply Excelsior Orthopaedics in western New York with digital x-ray, MR, and PACS technology. The vendor will also be providing its digital x-ray, MR, and PACS systems to TRIA Orthopaedic Center in Minnesota.
By AuntMinnie.com staff writers
February 24, 2005
Related Reading
GE lands Intermountain deal, February 18, 2005
GE adds to IT offerings, February 16, 2005
GE, PatientKeeper partner, February 1, 2005
GE Healthcare's 2004 sales jump 32%, January 25, 2005
GE displays HDMR cardiac techniques, January 24, 2005
Copyright © 2005 AuntMinnie.com