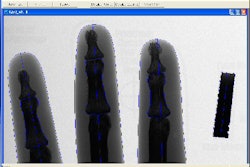
Surgical imaging developer Breakaway Imaging has received Food and Drug Administration 510(k) approval for its mobile O-arm imaging system.
The O-arm combines single and multiplanar 2D fluoroscopy with conebeam volumetric CT imaging. The system is mobile, able to access patients laterally, and controlled by robotics, according to the Littleton, MA-based firm.
Breakaway Imaging said it is initially targeting the device for use in orthopedic and spine procedures.
By AuntMinnie.com staff writers
May 13, 2005