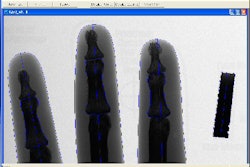
Digital radiography developer Swissray International has received Food and Drug Administration 510(k) clearance to market its newest DR system, the ddRCombi Trauma.
The device, designed for use in an emergency or trauma unit, features a fixed table with elevating base, four-way floating top, and automatic positioning of the detector for cross-table imaging with a wired or wireless handheld remote control, according to the Elizabeth, NJ-based vendor. The tabletop also slides away for access to the digital detector for wheelchair and upright exams, the company said.
ddRCombi Trauma also has fully programmable user functions, including automatic positioning for every body part, as well as from existing images via the company's SwissVision workstation. The system also has full remote monitoring capabilities.
By AuntMinnie.com staff writers
June 14, 2005
Related Reading
Swissray lands multiple-unit order, June 13, 2005
StorComm, Swissray get Washington install, January 19, 2005
Swissray adds Sutter Health order, January 12, 2005
Swissray, IDC reach settlement, January 5, 2005
Swissray scores legal win, December 20, 2004
Copyright © 2005 AuntMinnie.com