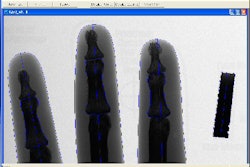
The Food and Drug Administration has cleared the DirectView DR 7500 digital radiography system from Eastman Kodak Health Group of Rochester, NY.
Kodak introduced the system at the 2004 RSNA meeting. The unit was designed to be a flexible system that can be adapted to the needs of imaging facilities of different sizes.
DirectView DR 7500 includes a wall stand capable of three-axis movement to capture upright, horizontal, and cross-table projections. It also includes a fixed elevating tabletop with four-way float for flexible patient positioning. An integrated operator console controls all imaging and generator functions.
Kodak will begin shipping the system in the fourth quarter of 2005.
By AuntMinnie.com staff writers
July 6, 2005
Related Reading
Kodak, BlueTie debut secure e-mail service, June 8, 2005
New CR paces Kodak SCAR news, June 2, 2005
Kodak adds RIS, PACS orders, May 26, 2005
Kodak debuts mammo PACS workstation, May 25, 2005
Jackman and Marsh named as Kodak VPs, May 13, 2005
Copyright © 2005 AuntMinnie.com