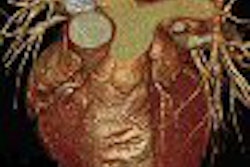
Multimodality vendor GE Healthcare of Chalfont St. Giles, U.K., has received Food and Drug Administration clearance for Innova CT, a new technique that enables the creation of 3D images on the company's Innova 3100 and 4100 angiography systems.
GE's Innova CT technology produces CT-like 3D volumes from rotational angiography acquisitions. The technology enables the imaging of bone and soft tissue with the same system, which should prove to be an advantage during interventional procedures that previously might have required CT scans for guidance, according to the company.
The Innova CT clearance follows up on the launch earlier this year of a 3D vascular imaging technology, Innova 3D, that enables users to better visualize low-contrast structures such as soft tissue and bone detail.
By AuntMinnie.com staff writers
September 7, 2005
Related Reading
GE gets U.S. Innova 2100IQ install, August 31, 2005
GE signs Novation, August 25, 2005
GE names Riesenberg to IT post, August 18, 2005
GE, Insightec ink new deal, August 17, 2005
GE adds to VHA contract, August 5, 2005
Copyright © 2005 AuntMinnie.com