CHICAGO - At this week's RSNA 2015 meeting, Toshiba America Medical Systems is highlighting new entry-level CT and ultrasound scanners, as well as a new multipurpose radiography/fluoroscopy system, a major upgrade for its 3-tesla MRI line, and an upgrade for its CT/angiography room.
CT
Aquilion Lightning is a highlight in the CT section of Toshiba's booth. Launched in Europe earlier in 2015, Lightning is being brought to North America to boost the company's presence in the entry-level CT segment.
Lightning is a 16-detector-row, 32-slice scanner designed to offer premium Toshiba technology in a package that can be sited in a small space. Technologies available on the system include the company's PureVision detector and SurekV automated tube current selection protocol, as well as its adaptive iterative dose reduction (AIDR) 3D technique.
Lightning will eventually replace the Aquilion RXL scanner at the entry-level price point; with Lightning, Toshiba has widened the gantry to 78 cm like the rest of its CT product line, compared with the smaller 72-cm gantry on RXL. The new system comes standard with 0.6-second rotation, with 0.5-second rotation available as an option.
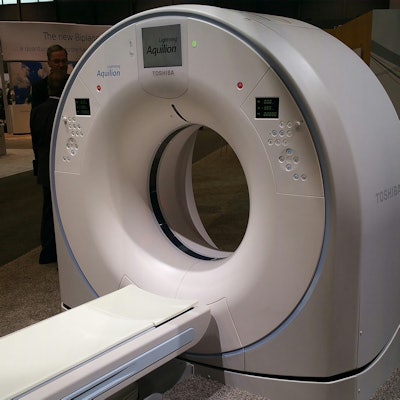 Toshiba's Aquilion Lightning.
Toshiba's Aquilion Lightning.Lightning has been cleared for use with a 42-kW x-ray generator, but Toshiba has submitted documentation to the U.S. Food and Drug Administration (FDA) for a more powerful 50-kW generator for the U.S. market.
On the company's flagship Aquilion One scanner, Toshiba has introduced forward-projected model-based iterative reconstruction (FIRST), a new protocol that will be available as an option. FIRST offers slightly better dose reduction than AIDR 3D, but the protocol's real benefit is its speed and ease of use, according to the company, with reconstruction times of three minutes for volume-based CT scans.
One FIRST site has been installed at the U.S. National Institutes of Health for the past month, and the technique received FDA clearance the day before RSNA 2015 opened for body and lung applications, with cardiac and musculoskeletal applications to follow. When FIRST begins shipping in January, AIDR 3D will still be available for Aquilion One scanners.
Last but not least, Vitality XT is a new dose-management program that gives users more control over changes to their CT protocols. A number of radiation dose incidents have occurred after scanning protocols were changed from factory settings; in a few cases, the protocols ending up delivering radiation overdoses to patients, which weren't discovered until months later.
Vitality XT is designed to prevent such occurrences by alerting radiology managers and requiring their approval whenever a protocol has been changed. The software can even be set so that a protocol change can't be used until it's been approved. Vitality XT is now shipping for Toshiba CT scanners using version 7 of its software, including Aquilion One, Prime, and Lightning models.
X-ray and interventional
In x-ray's classic modality, Toshiba is emphasizing Ultimax-i FPD, a multipurpose tilting radiography/fluoroscopy (R/F) unit that is designed to also perform interventional procedures that previously required a mobile C-arm.
Ultimax-i FPD utilizes a 17 x 17-inch digital detector and elevating 90°/90° table. It is designed to fit into a small space while offering the versatility to perform a wider variety of clinical procedures.
Whereas in the past clinicians might have rolled a portable C-arm into the room for special procedures, Ultimax-i FPD is designed to perform some of these applications itself, such as placements of filters and central lines and kyphoplasty and vertebroplasty procedures. It also performs procedures for which a tilting table might be needed, such as myelograms, swallow studies, esophageal exams, and barium studies.
Ultimax-i FPD has a 500-lb table capacity, and Toshiba began selling the system in the U.S. on October 1 after receiving FDA clearance.
Meanwhile, Toshiba's Infinix 4DCT interventional configuration is getting an upgrade with a more powerful CT scanner, with the new offering known as Infinix 4DCT Vision.
 Infinix 4DCT Vision.
Infinix 4DCT Vision.Infinix 4DCT pairs a CT scanner with an angiography system in a single room to allow both modalities to be used during interventional procedures. First launched with Toshiba's Aquilion Prime CT system, Infinix 4DCT is now available with the company's flagship Aquilion One Vision system.
Using a Vision scanner in the configuration puts that system's 16 cm of coverage per gantry rotation at the disposal of users, according to Toshiba. This enables the acquisition of an entire organ, such as the liver, in one 0.275-second rotation.
At RSNA 2015, Toshiba is emphasizing oncology applications for Infinix 4DCT Vision, such as whole-organ perfusion, now possible because of the wide detector. The flexibility of the configuration also allows interventional physicians to plan, treat, and verify the treatment of patients, all in one setting.
Several Infinix 4DCT Vision systems have been installed globally, with the first U.S. unit shipping in January.
Ultrasound
In ultrasound, Toshiba is highlighting the modality's exemplary safety profile, promoting applications that the company believes will improve the diagnostic confidence of users of its products.
The company's main product launch in ultrasound at RSNA 2015 is Xario 100, a new midrange, 64-channel, shared-services scanner that Toshiba is bringing to the U.S. after a year of selling it in Europe and Asia.
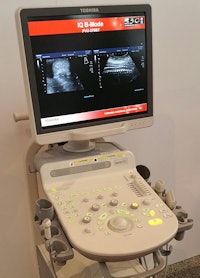 The Xario 100 ultrasound scanner.
The Xario 100 ultrasound scanner.Xario 100 supports radiology applications such as gastrointestinal imaging, obstetrics/gynecology, and 4D imaging, as well as full cardiology and pediatric cardiology applications. The system is designed to address a recent trend in the market to have midrange products covering a wide range of clinical applications, according to the company.
Xario 100 joins the 128-channel Xario 200 in the company's line of midrange ultrasound scanners in the U.S.; the new system has FDA clearance. For Xario 200, Toshiba is introducing a new version 4 software release at RSNA 2015 that includes features for pediatric imaging such as pediatric heart and neonatal head exams.
On the software side, Toshiba is launching a new software upgrade called Innovation 2016 for its Platinum platform, which is available on the Aplio 300 and Aplio 500 systems.
Platinum was introduced at the RSNA 2014 show, and it brought advanced applications such as Superb Micro-Vascular Imaging (SMI) and Smart Navigation to Aplio systems. With Innovation 2016, Toshiba is offering multiple new enhancements.
For example, support for 3D imaging has now been added to SMI as well as Toshiba's shear-wave elastography protocol, giving the company's customer base access to 3D shear-wave elastography. The company has also added new maps and other features to improve the diagnostic confidence of shear-wave users.
Other new features of Innovation 2016 include support for fusing ultrasound images with MRI for prostate imaging, as well as a new 11-Mhz transducer. The package will begin shipping in December, after RSNA 2015. All new systems will ship with the software, and users in the field will be offered an upgrade path.
Finally, Toshiba is highlighting an interventional 4D probe, as well as a 2D endovaginal probe with a longer handle.
MRI
New hardware and software upgrades are on order in the MRI section of Toshiba's booth.
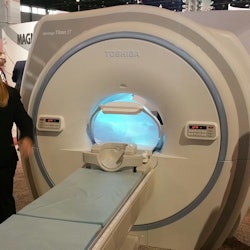 Toshiba is showing a new patient comfort experience for its MRI scanners as a work in progress.
Toshiba is showing a new patient comfort experience for its MRI scanners as a work in progress.The hardware upgrade, called Vantage Titan 3T/iS Edition, consists of an optional version of Toshiba's high-amplitude Saturn gradients, rated with a maximum amplitude of 45 mT/m and a slew rate of 203 mT/m/msec.
The software upgrade is a new version 3.5 of Toshiba's M-Power software, which includes new features, pulse sequences, and applications. This includes ultrashort TE (UTE), which lets users image tissues with relaxation times as short as 96 microseconds, making it well-suited for tissues such as the lung that are usually difficult to image with MR. Other sequences include MPRAGE, enhanced low-spoiled black-blood for enhanced vessel visibility, T2 and T2* mapping, and double spin echo.
All new Toshiba MRI scanners will ship with M-Power 3.5, while users in the field can upgrade if they wish. Toshiba is also pointing out that M-Power 3.5 runs on Windows 7, making it compliant with purchasing rules from the U.S. military.
Toshiba is also talking up FDA clearance for version 3.1 of the operating software for its Vantage 1.5 Titan scanner. The upgrade includes all applications and sequences first launched on the 3-tesla scanner last year, according to the company.
Finally, Toshiba is demonstrating a patient comfort experience in which soothing images are projected onto a screen that the patient can watch as he or she moves into the magnet bore.