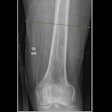
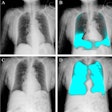
Compañía Mexicana de Radiología (CMR) as received U.S. Food and Drug Administration (FDA) clearance for its ARiX RAD radiographic system.
The system is intended for applications such as extremity, head, abdominal, and thoracic imaging. It includes a control console that features an anatomical programmer aimed at rapid and accurate radiographic technic selection designed to increase the speed of examinations with a goal of increasing productivity.
Configuration of the ARiX RAD system can be achieved with one or more digital detectors.
CMR's portfolio of products, including x-ray imaging, information technology, and molecular breast imaging, is being exhibited at RSNA 2019 in Chicago.