The next time a sympathetic soul tells you that he "feels your pain," you should feel free to correct him: The experience of pain is complex and highly individualized, according to researchers in Michigan who have pioneered the use of PET to study the effects of pain on the brain.
"The utilization of functional neuroimaging techniques measuring brain metabolic responses to pain under various conditions is providing information on the various circuits in the human brain involved in these processes," said Dr. Jon-Kar Zubieta, Ph.D., during a presentation at the 2002 American Association for the Advancement of Science in Denver. Zubieta is an associate professor of psychiatry and radiology at the University of Michigan School of Medicine in Ann Arbor.
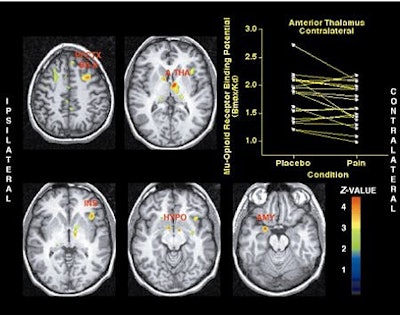 |
| Activation of the µ-opioid receptor system during sustained masseter muscle pain. Brain areas where significant reductions in regional µ-opioid receptor availability from placebo to sustained pain were obtained. Z-scores of statistical significance are represented by the pseudocolor scale on the right of the image, and are superimposed over an anatomically standardized MRI image in axial views. Image data were prepared so that the left side of the image corresponds to the side where the painful stimulus was applied (ipsilateral). PFCTX BA 8 = prefrontal cortex, Brodmann areas 8/9; A THA = anterior thalamus; INS = anterior insular cortex; HYPO = hypothalamus; AMY= amygdala. Image courtesy of Dr. Jon-Kar Zubieta. |
"The view of pain as a relatively simple stimulus eliciting largely automatic responses is contradicted by the growing recognition that its significance is complemented by individualized affective, cognitive, and motivational processes," he continued. "In turn, these interpretative processes appear capable of modifying the function of the very pathways and neurochemical systems originally involved in the perception of pain. The challenge becomes to reverse-engineer the more complex human responses to pain into components that can be studied at the molecular and cellular level, in effect completing a full circle."
Zubieta and his team at the university’s Mental Health Research Institute have found significant indications of what individualizes a painful experience, such as gender, hormones (estrogen, testosterone), and genes.
Specifically, the team used PET scans to study a major natural painkiller system in the brain, the µ-opioid neurotransmitter system. This system controls the pain-killing effect of the endogenous opioid brain chemicals, endorphins, or enkephalins.
Using a radioactive tracer attached to a molecule that binds only to µ-opioid receptors, the researchers performed PET scans on 20 healthy subjects (13 men, 7 women). In a double blind, placebo-controlled study, sustained, induced pain was measured with PET, which monitored the µ-opioid neurotransmitter system (Science, July 13, 2001).
To create the tracer, short-lived radioactive carbons atoms were attached to a molecule that bound only to µ-opioid receptors. The decay signals allowed the team to measure the release of endogenous opioids and the activation of the µ-opioid receptors.
While in the PET scanner, a prolonged jaw pain, similar to temporo-mandibular joint (TMJ) disorder, was induced in the subjects by injecting high-concentration salt water into the jaw muscle. The solution was administered for 20 minutes to each subject.
The volunteers were asked to rate their intensity of pain every 15 seconds. A computer program, which controlled the intensity of the pain stimulus, compiled their ratings. Afterward, the volunteers completed a questionnaire about how the experience made them feel.
The results showed response that was strongest in the brain regions where sensation and emotion are rooted, a response tied directly to the ratings of the pain experience that the volunteers gave, Zubieta explained.
"We saw an intense activation of the µ-opioid system in areas such as the amygdala, the thalamus, the hypothalamus, the frontal cortex, and the nucleus accumbens, as much as a 12% change over baseline conditions," he said. "And the higher the level of activation, the lower the scores the volunteers gave for pain-related sensations and emotions like feelings of the unpleasantness of pain." The regions most significantly affected were exactly those involved in the affective, or emotional, responses, and those primarily involved in processing sensations.
The results also showed wide individual variations in the intensity of the brain anti-pain response, which correlated with the individual’s sensory and affective responses to the pain experience.
The activation of the anti-pain response was dramatic in some volunteers when the placebo and pain-inducing conditions were compared, while in others the response was much less pronounced. And those who had the biggest change tended to rate the experience of pain, both in its sensory and emotional aspects, the lowest.
"This may help explain why some people are more sensitive, or less sensitive, than others when it comes to painful sensations," Zubieta said. "We show that people vary both in the number of receptors that they have for these anti-pain brain chemicals, and in their ability to release the anti-pain chemicals themselves. Both of these factors appear to determine the emotional and sensory aspects of a painful experience."
"Such variability in the pain-response system may help explain why some people react to pain and pain medications differently. It may also be quite relevant to why some people, but not others, develop chronic pain conditions," he added.
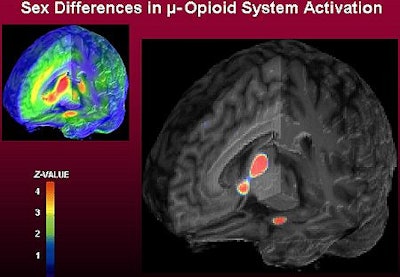 |
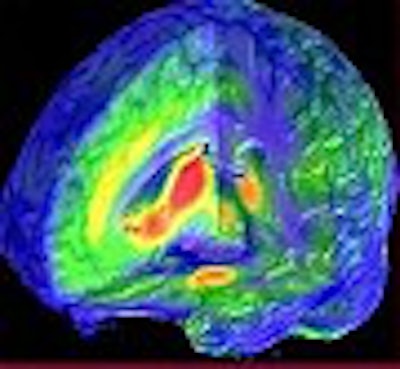
| Left: Three dimensional representation of µ-opioid receptor binding potential values in a representative healthy volunteer, superimposed over an MRI image standardized to ICBM stereotactic coordinates. Binding potential values (1-4) are also represented by the pseudocolor scale in the lower part of the figure. Right: Brain areas where significantly larger magnitudes of µ-opioid system activation were observed in males, compared to females during their early follicular phase. These included the nucleus accumbens, amygdala, ventral pallidum and thalamus. Z scores of statistical significance are represented by the pseudocolor scale on the left of the figure, and are superimposed over an anatomically standardized MRI image in 3-D views. Image courtesy of Dr. Jon-Kar Zubieta. |
Zubieta’s team is now conducting PET scans with another mixed-gender cohort of subjects to determine how estrogen levels affect the pain experience. Thus far, they have found that when estrogen levels are high, the µ-opioid receptors respond actively to pain, releasing endorphins to diminish its effect. When estrogen was low, the µ-opioid was less effective in reducing pain.
"The interplay of gender, hormones, genetics, and brain neurochemistry appears to induce our individual response to pain. All of this work is helping tell us how important individual differences are in the experience of pain and other significant stressors," Zubieta said. "Our findings underlie the need to think about pain, particularly prolonged or sustained pain, as the result of complex interfaces between injury and our own capacity to regulate its severity and significance."
By Bruce SylvesterAuntMinnie.com contributing writer
March 12, 2003
Related Reading
Discography with gadolinium works for iodine-allergic patients, February 24, 2003
Functional MRI shows how brain lets you smell that smell, February 14, 2003
Metabolic responses in the brain may predict lethality of suicide attempts, January 29, 2003
Brain scans document fibromyalgia pain, June 18, 2002
Copyright © 2003 AuntMinnie.com