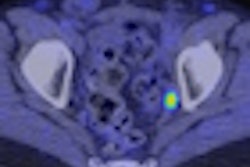
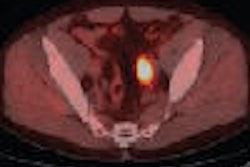
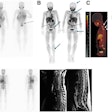
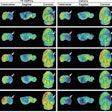
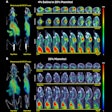
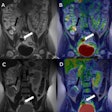
The U.S. Food and Drug Administration (FDA) has approved the production and use of PET radiopharmaceutical carbon-11 (C-11) choline injection to detect recurrent prostate cancer, the agency announced.
PET imaging with choline C-11 is indicated for patients whose blood prostate-specific antigen (PSA) levels are increasing after earlier treatment for prostate cancer, according to the FDA.
The agent must be produced in a special facility and administered to patients shortly after its production. While PET imaging with choline C-11 has been performed at a few facilities over the past several years, none of these sites was approved by the FDA to manufacture the agent.
However, the FDA has now approved the Mayo Clinic PET Radiochemistry Facility in Rochester, MN, to produce choline C-11, the FDA said.