By attaching gallium-68 (Ga-68) to a fibroblast activation protein molecule inhibitor, German researchers have achieved greater accuracy with PET/CT in staging patients with pancreatic ductal adenocarcinoma (PDAC), according to a study published online October 23 in the Journal of Nuclear Medicine.
PDAC consists of cancer cells that express fibroblast activation protein (FAP). By adding a FAP inhibitor (FAPI) labeled with gallium-68 to PET/CT imaging, more than half of the PDAC patients in this study had their cancer more accurately staged or restaged, compared with results from contrast-enhanced CT.
"PDAC is clinically challenging with very high mortality rates. Improvement in survival can only be achieved by effective treatment approaches customized to the individual patient's disease status," wrote the authors, led by Dr. Manuel Röhrich and colleagues from Heidelberg University Hospital and German Cancer Research Center (DKFZ). "Thus, hybrid imaging using [a] FAPI-tracer may open up new applications in staging and restaging of PDAC."
Patients with PDAC have only a 10% chance of survival after five years. While FDG-PET/CT has shown a propensity to predict and assess how well PDAC patients respond to treatment, FDG is "clearly not an ideal imaging agent for PDAC due to its variable detection of metastatic lymph nodes and possible false-positive findings in inflammation," the authors wrote.
By comparison, recent studies have shown FAP to be a "promising new target molecule for PET imaging of various epithelial tumors, among them PDAC."
When combined with Ga-68-PET/CT, Ga-68-labeled FAP inhibitors advance the hybrid modality to show the interaction between cancer and tissue cells, which promotes the growth of tumors. By doing so, it provides an alternative to morphological or metabolic imaging, which cannot visualize this process.
To further explore the potential of Ga-68-FAPI-PET/CT in tumor, node, and metastases (TNM) staging versus contrast-enhanced CT, Röhrich and colleagues retrospectively analyzed 19 patients (median age, 64 years; range, 52-80 years). The subjects included 12 patients with progressive or recurrent PDAC and seven patients with primary PDAC.
PET scans were performed 60 minutes after administration of 150-250 MBq of Ga-68-labeled FAP-specific tracers. CT scans for TNM staging were performed an average of 17.6 days before FAP-specific PET/CT (Biograph mCT Flow, Siemens Healthineers).
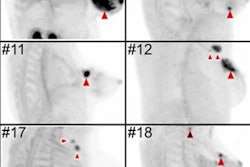
The researchers found that Ga-68-FAPI-PET/CT provided new TNM findings for 10 of 19 PDAC patients (53%), which included the upstaging of eight of 12 patients (67%) with recurrent or progressive disease, while one patient's (8%) recurrent or progressive cancer was downgraded. Among seven PADC patients with primary disease, one case out of seven (14%) was upstaged; no patients were downstaged.
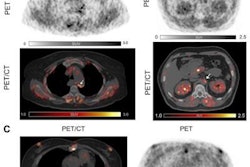
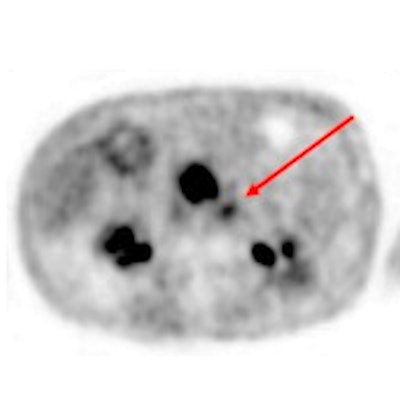
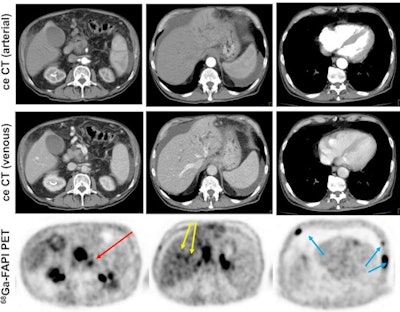 Contrast-enhanced CT (ceCT) and FAPI-PET/CT (68Ga-FAPI-PET) images from the same patient with local disease recurrence. In contrast to CT, FAPI-PET/CT discriminates a metastatic lymph node from the local recurrence mass (red arrow). FAPI-PET/CT also reveals possible new liver (yellow arrows) and bone (blue arrows) metastases. Images courtesy of Journal of Nuclear Medicine.
Contrast-enhanced CT (ceCT) and FAPI-PET/CT (68Ga-FAPI-PET) images from the same patient with local disease recurrence. In contrast to CT, FAPI-PET/CT discriminates a metastatic lymph node from the local recurrence mass (red arrow). FAPI-PET/CT also reveals possible new liver (yellow arrows) and bone (blue arrows) metastases. Images courtesy of Journal of Nuclear Medicine."In all cases, changes in staging were caused by the detection of new or additional distant metastases in one or more organ systems," the authors added. They also found a "markedly elevated uptake" of Ga-68-FAPI in most PDAC patients as soon as one hour after administration.
The findings suggest that Ga-68-FAPI- PET/CT is a "promising new imaging modality in staging of PDAC that may help to detect new or clarify inconclusive results obtained by standard CT-imaging," Röhrich and colleagues concluded.
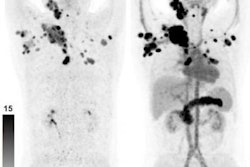
"Analyses of tracer biodistribution demonstrated a high FAPI uptake in primary PDAC as well as lymph nodes and distant metastases, whereas healthy tissues have negligible background activity," they wrote. "This leads to excellent tumor/background ratios for PDAC, similar to those shown by previous studies on FAPI-PET/CT in PDAC and other tumors."
Disclosure note: Study co-authors Drs. Uwe Haberkorn, Clemens Kratochwil, and Frederik Giesel have filed a patent application for quinoline-based FAP targeting agents for imaging and therapy in nuclear medicine.