Overlooking imaging costs related to the controversial Alzheimer's disease drug aducanumab (Aduhelm) prior to its approval was a mistake that could ultimately threaten Medicare's solvency, according to an analysis published January 14 in JAMA Health Forum.
The researchers estimated that the annual cost for Medicare to cover aducanumab (Aduhelm, Biogen) for 25% of eligible U.S. patients with Alzheimer's disease could be as high as $37.4 billion, with a significant stake spent on MRI monitoring of patients.
"Preliminary U.S. spending estimates either used extrapolated [Alzheimer's disease] dementia prevalence data from 2012 or did not explicitly quantify ancillary costs, such as additional diagnostic imaging," wrote lead author Dr. John Mafi, an associate professor of medicine at University of California, Los Angeles in a research letter.
Aduhelm was approved by the U.S. Food and Drug Administration in June 2021 under an accelerated regulatory pathway, a move that was controversial both due to the high cost of Aduhelm as well as the equivocal nature of the data supporting the drug's effectiveness.
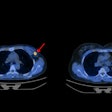
Aside from the cost of the drug ($56,000 per year, although Biogen dropped it to $28,200 after insurance companies balked), preliminary estimates didn't account for monitoring patients for amyloid-associated imaging abnormalities (ARIAs), which can occur in up to 41% of patients treated with Aduhelm, according to the authors.
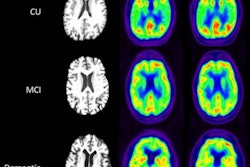
ARIAs comprise a spectrum of imaging findings detected on brain MRI scans. Research has shown they are associated with the investigational use of monoclonal antibodies, including Aduhelm, in patients with Alzheimer's disease. Symptoms of ARIAs are related to cerebral edema and include headaches, vomiting, confusion, and gait disturbance.
In this cross-sectional study, Mafi and colleagues identified 8,396 participants from the 2016 Health and Retirement Study, a nationally representative survey of older adults, representing approximately 41.2 million U.S. adults 65 or older with Medicare coverage.
Based on percentages of amyloid-positive patients with mild cognitive impairment (MCI) or dementia reported in population studies, the researchers used 37% for a low estimate and 68% for an upper estimate and based on the percentages calculated that between 1.1 million to 5.7 million Medicare beneficiaries could be potentially eligible to receive treatment with Aduhelm.
The group used pooled data from Aduhelm trials showing a moderate to severe ARIA rate of 26% and a symptomatic mild ARIA rate of 3.6%. With an average duration of 12 weeks, each ARIA would require three additional MRI scans, the authors wrote.
Total annual upper estimated payment by Medicare for brain MRI scans in patients receiving Aduhelm could reach $1 billion, according to the study. In all, additional imaging and other ancillary health services related to aducanumab comprised nearly 20% of Medicare spending estimates, the authors wrote.
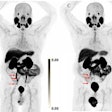
The group included diagnostic amyloid PET cost estimates for identifying patients eligible for treatment with Aduhelm. Medicare's bill for PET scans on the upper end was estimated at $2.6 billion.
"The drug and ancillary costs of aducanumab could seriously strain Medicare's budget, raising questions of how to maximize the value of public dollars, particularly for a drug with unknown benefit and known risk of patient harm," the researchers concluded.
The analysis coincided with news on January 12 that the U.S. Centers for Medicare and Medicaid Services (CMS) has proposed a national coverage determination (NCD) that would limit coverage for drugs like aducanumab only to people enrolled in qualifying clinical trials.
"This proposed [NCD] is the result of robust evidence analysis conducted through a thorough review process that found while there may be the potential for promise with [Aduhelm], there is also the potential for harm to patients," said CMS Administrator Chiquita Brooks-LaSure in a press release.
The CMS is expected to announce its final decision on the proposed NCD by April 11, 2022.