Amyloid PET imaging performed in just a few minutes appears equal to standard FDG-PET imaging for detecting glucose hypometabolism in Alzheimer's disease patients, according to a study published January 18 in the European Journal of Hybrid Imaging.
"Early-frame amyloid scans could replace F-18 FDG scans in the measurement of cerebral glucose metabolism," wrote corresponding author Alison Myoraku of Stanford University and colleagues of the international Alzheimer's Disease Neuroimaging Initiative (ADNI).
The finding could have an impact reducing costs due to multiple PET scans, as well as radiation exposure in patients, the authors wrote.
For more than 20 years, FDG-PET has been used to measure metabolic glucose activity in the brains of Alzheimer's disease patients. Studies have shown that changes in glucose activity occur early in the disease and can predict diagnosis.
Similarly, F-18 florbetapir imaging (amyloid PET) is a well-established method for diagnosing patients with Alzheimer's disease based on amyloid plaque deposits in the brain, another hallmark of the disease. Lab studies have shown that F-18 florbetapir PET imaging may also provide clues on glucose hypometabolism based on tracer uptake through various regions in the brain.
But acquiring information on both of these aspects of the disease requires multiple PET scans, which is expensive and exposes the patient to multiple doses of radiation, the authors wrote.
In this study, the researchers investigated to what extent the information captured in early frames of amyloid PET scans compared to that of F-18 FDG PET scans among cognitively unimpaired patients (CU), patients with mild cognitive impairment (MCI), or patients with dementia.
The group culled data on 100 patients from the ADNI database. Participants had both early-frame F-18 florbetapir PET and F-18 FDG PET scans less than 2.5 years apart. The group was composed of 52 cognitively unimpaired individuals, 33 with MCI, and 15 with dementia.
The FDG group was injected with 185 mBq (5 mCi) of F-18 FDG and had a standard dynamic 3D scan consisting of six 5-minute frames acquired 30 to 60 minutes after injection. Early-frame F-18 florbetapir images were acquired between 45 seconds and 6 minutes, as recommended in previous studies, the authors wrote.
Standardized uptake values for 82 regions of interest (ROIs) were then extracted and compared between the PET images. The brainstem served as a reference region for both the early-frame F-18 florbetapir images and F-18 FDG images to estimate the regional standardized uptake value ratio (SUVR).
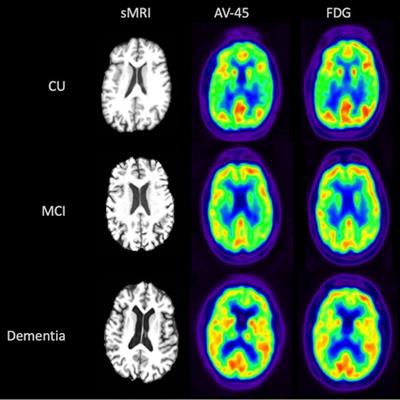
 Visual comparison of sMRI, early-frame F-18 florbetapir, and F-18 FDG-PET for participants from each disease state (CU, MCI, and dementia). Image courtesy of the Journal of Hybrid Imaging.
Visual comparison of sMRI, early-frame F-18 florbetapir, and F-18 FDG-PET for participants from each disease state (CU, MCI, and dementia). Image courtesy of the Journal of Hybrid Imaging.The partial correlation of early-frame F-18 florbetapir with F-18 FDG was significant in all 84 brain ROIs, with correlation values ranging from 0.61 to 0.94, the researchers found. The highest correlation between the early-frame F-18 florbetapir and F-18 FDG data within cortical regions was in the anterior temporal lobe (left: r = 0.85, p < 2e-16; right: r = 0.85, p < 2e-16).
The average F-18 FDG SUVR for the whole brain was 1.21, while the average early-frame F-18 florbetapir SUVR was 1.07, according to the findings.
"The regional uptake measurements from early-frame F-18 florbetapir are strongly correlated with regional glucose metabolism as measured in ground-truth F-18 FDG-PET scans, regardless of disease state," the researchers wrote.
Overall, this study may have significant implications for researchers studying biomarkers related to glucose metabolism in Alzheimer's disease in a cohort of participants with a wide range of clinical stages, the researchers suggested.
"This finding could be extremely beneficial in the context of a clinical trial that recruits across the [Alzheimer's disease] spectrum and uses glucose metabolism as a measure of either treatment efficacy or illness progression," the authors wrote.
Ideally, however, it would be important to identify whether changes in glucose metabolism over time could be detected in early-frame amyloid PET scans as they are in F-18 FDG scans, and the researchers noted the limitation of exploring correlations in this study in a cross-sectional cohort.
"Future studies should focus on longitudinal early-frame amyloid PET imaging studies to further assess the value of early-frame imaging as a marker of brain metabolic decline," the authors concluded.