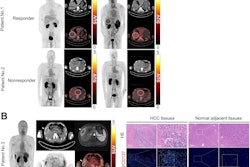
Zirconium-89 (Zr-89) rituximab PET imaging shows promise for identifying whether patients with autoimmune lung disease may respond to immunotherapy treatment, according to a group in the Netherlands.
Researchers led by Dr. Human Adams of St. Antonius Hospital in Nieuwegein found that the imaging technique correlated significantly with clinical outcomes in patients after they began anti-CD20 therapy (rituximab) treatment. The study was the first phase II clinical trial testing the approach in patients.
"If a noninvasive biomarker could measure the presence of CD20 cells in the lungs, this would potentially be of use to distinguish clinical responders from nonresponders before treatment is started," Adams and colleagues wrote. The study was published February 24 in the European Journal of Nuclear Medicine and Molecular Imaging.
Immune-mediated inflammatory diseases (IMID) with interstitial pneumonitis (IP) are a group of diseases that cause scarring of lung tissue and can make the lungs stiff and inelastic. IMID-IP may be treated with drugs such rituximab, which destroys the abnormal B cells in the lungs driving the disease. However, the clinical response to the drug is variable, the authors wrote.
Zr-89 rituximab is a PET radiotracer designed to bind to CD20 receptors on the B cells and thus can reveal varying levels of these "target" cells on PET scans. In a previous study, the authors demonstrated the safety of the approach. This study was conducted to further evaluate it in a group of prospective patients.
"This is the first prospective trial to investigate the predictive potential of this novel imaging technique in IMID-IP and to compare imaging data with clinical outcome after rituximab treatment," the authors wrote.
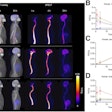
The researchers recruited 21 patients with IMID-IP with deteriorated pulmonary function. Patients had not been treated with immunotherapy previously and received a dose of 1,000 milligrams rituximab on day one. PET/CT was performed on days three and six, with levels of radiotracer uptake (standard uptake values, SUV) by B cells calculated for each patient.
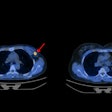
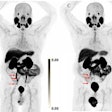
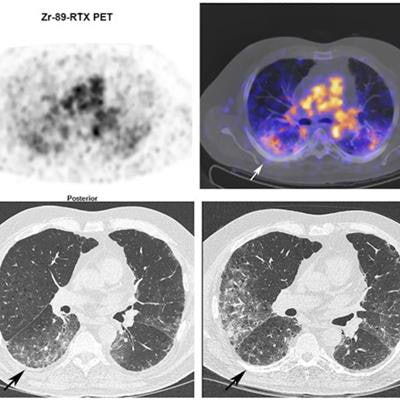
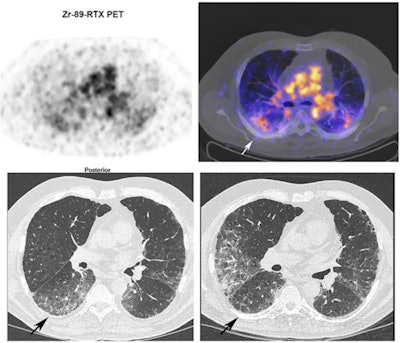 Zr-89 rituximab PET/CT and HRCT of a 65-year-old patient with fibrotic nonspecific interstitial pneumonia-associated rheumatoid arthritis (case 3). Zr-89 rituximab PET (top left) axial PET image (top right) fused Zr-89 rituximab PET/CT. HRCT (bottom left) at baseline of PET and HRCT after one year (bottom right). Matching of the axial view between the PET/CT and HRCT is not exactly possible since the HRCT is performed with an inspiration command and the PET/CT is in the resting state. The Zr-89 rituximab activity is more focused in the lower lobes. This central area on Zr-89 rituximab correlates more with the HRCT ground glass; see, for example, the right lower lobe ground-glass area. The HRCT after one year post rituximab did not show any ground glass in this area. However, new ground-glass areas emerged on the HRCT in the lower upper lobes. The patient remained stable in pulmonary function. Image courtesy of the European Journal of Nuclear Medicine and Molecular Imaging through CC BY 4.0.
Zr-89 rituximab PET/CT and HRCT of a 65-year-old patient with fibrotic nonspecific interstitial pneumonia-associated rheumatoid arthritis (case 3). Zr-89 rituximab PET (top left) axial PET image (top right) fused Zr-89 rituximab PET/CT. HRCT (bottom left) at baseline of PET and HRCT after one year (bottom right). Matching of the axial view between the PET/CT and HRCT is not exactly possible since the HRCT is performed with an inspiration command and the PET/CT is in the resting state. The Zr-89 rituximab activity is more focused in the lower lobes. This central area on Zr-89 rituximab correlates more with the HRCT ground glass; see, for example, the right lower lobe ground-glass area. The HRCT after one year post rituximab did not show any ground glass in this area. However, new ground-glass areas emerged on the HRCT in the lower upper lobes. The patient remained stable in pulmonary function. Image courtesy of the European Journal of Nuclear Medicine and Molecular Imaging through CC BY 4.0.Then, prior to and six months after treatment, the researchers compared the radiotracer uptake with pulmonary function tests (PFT) such as forced vital capacity (FVC), which were used to classify patients as reponders or nonresponders.
Fifteen patients (71%) were classified as responders. Pulmonary Zr-89 rituximab PET SUVmean was significantly correlated with change in patient FVC (K = 0.49) when using target-to-background ratios, but not when using SUVmean alone, the researchers found. In addition, Zr-89 rituximab SUVmean was significantly higher in responders than in nonresponders, according to the findings.
"A higher pulmonary uptake of Zr-89 rituximab correlated with improvement of PFT and treatment outcome," the group wrote.
Ultimately, the authors noted that the use of rituximab in these patients is offlabel. Rituximab treatment in patients with IMIDs with progressive life-threatening ILD represents a promising rescue option, given that 71% of patients responded in this trial, they noted.
In addition, the study showed that a novel radiolabeled anti-CD20 imaging technique might be useful as a noninvasive predictive biomarker, they wrote.
"Our data showed a higher pulmonary CD20 presence in responders compared to nonresponders when treated with rituximab and therefore warrant further study into this novel imaging technique," the authors concluded.