FAPI-PET imaging can predict how patients with unresectable hepatocellular carcinoma (uHCC) may respond to immunotherapy drugs, according to a study published July 27 in the Journal of Nuclear Medicine.
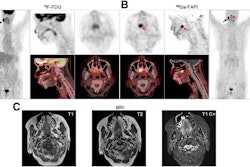
A group led by Meiqi Wu, MD, of the Chinese Academy of Medical Science and Peking Union Medical College Beijing, studied gallium-68 (Ga-68) fibroblast activation protein inhibitor (FAPI) PET/CT baseline scans in patients who subsequently went on a combination of immunotherapy drugs. They found that the FAPI-PET scans predicted which patients responded to treatment.
"Baseline Ga-68 FAPI-PET/CT may facilitate selection of uHCC patients for the combination of [immune checkpoint blockade] and targeted therapy," the group wrote.
Pharmaceutical advances have changed the therapeutic landscape uHCC, according to the authors. In particular, the combined use of immune checkpoint blockade inhibitors and multikinase inhibitors has achieved remarkable successes for treating these patients, they wrote.
However, these treatments are expensive, and only a subportion of patients with uHCC appear to benefit from the therapies. An effective way to determine which patients may or may not respond could be a game changer, they noted.
Thus, in this study, the researchers analyzed findings in 22 patients with uHCC who underwent baseline FAPI-PET/CT scans after they began taking a combination of an immune checkpoint blockade inhibitor and lenvatinib (a multikinase inhibitor).
Between July 2020 and April 2022, patients underwent FAPI-PET/CT scans, with combination immunotherapy initiated within two weeks after the scans. Patients were followed up regularly, on average every two months over a period of 16.5 months. Eleven patients experienced durable clinical benefits. For the entire cohort, the median progression-free survival was 4.8 months and the median overall survival was 14.4 months.
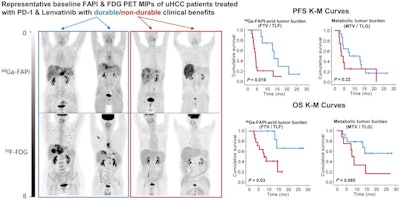 A graphical abstract. Image courtesy of the Journal of Nuclear Medicine.
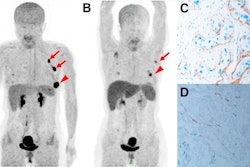
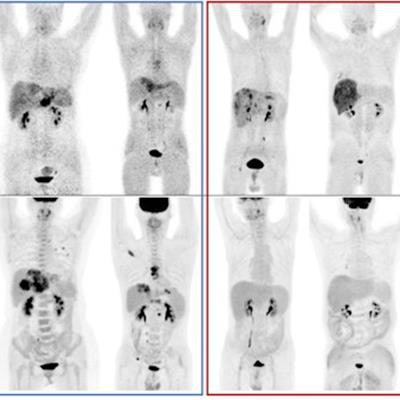
A graphical abstract. Image courtesy of the Journal of Nuclear Medicine.According to the findings, patients who did not respond to the treatments showed a significantly higher FAPI-avid tumor measurements than those who responded. Nonresponders with high FAPI-avid tumor burden had significantly shorter progression-free survival (4 months vs. 13.5 months). In addition, patients with a high FAPI–avid tumor burden showed a significantly shorter overall survival (7.8 months vs. not reached).
"Volumetric indices on baseline Ga-68 FAPI-PET/CT were potentially independent prognostic factors to predict durable clinical benefit," the authors wrote.
Ultimately, this study adds to growing evidence that Ga-68 FAPI-PET/CT shows potential to improve care in patients with advanced, inoperable liver cancer, undergoing new immunotherapy treatments, they wrote.
"The value of Ga-68 FAPI-PET should be further investigated in phase III uHCC clinical trials," the group concluded.
A link to the full study can be found here.