A group in England has established PET imaging as a new approach for studying gait – an excellent indicator of physical, emotional, and mental health, according to a study published February 6 in NeuroImage.
In a proof-of-concept study, the researchers used F-18 FDG-PET scans in healthy participants within minutes after they performed standing and walking tasks and identified specific changes in brain glucose metabolism. The findings could open new avenues for research in patients with gait impairment related to conditions like Parkinson’s disease, noted lead author Hilmar Sigurdsson, MD, of Newcastle University.
“We have established the feasibility and tolerability of a novel method capable of capturing neural activations related to actual walking,” the group wrote.
Imaging to detect brain activation related to walking is commonly performed using functional MRI (fMRI), with subjects in recent studies depressing foot pedals to simulate gait movements, for instance. However, these approaches may miss key information related to actual walking, the authors noted.
F-18 FDG-PET scans allow clinicians to measure the brain's energy demands based on glucose metabolism. In these scans, injected F-18 FDG radiotracer (a glucose analog) is taken up by cells similar to glucose and thus reveals areas in the body of increased metabolic activity.
In this study, the researchers hypothesized that F-18 FDG administered intravenously in participants prior to walking would reveal brain gait metabolism in subsequent PET scans.
The group enrolled 15 healthy older adults (10 females, age range: 60.5-70.7 years old) with normal cognition from the community. Each participant received intravenous injections of F-18 FDG before engaging in two distinct tasks.
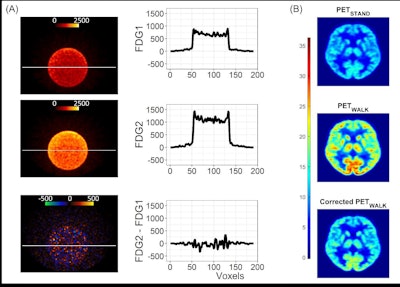 Data analysis validation. Panel A: Image and line profile through the bottle on scans 1 (top) and 2 (middle), and the difference image (bottom) calculated according to Equation 3. Panel B: Data from a representative participant showing the effect of the dose correction on the PETWALK image using Equation 1 when applied to co-registered images in native subject space. The images were then warped to standard Montreal Neurological Institute (MNI) space Top: PETSTAND, middle – uncorrected PETWALK, bottom – corrected PETWALK. The color bar shows image intensity across the three images. Image courtesy of NeuroImage.
Data analysis validation. Panel A: Image and line profile through the bottle on scans 1 (top) and 2 (middle), and the difference image (bottom) calculated according to Equation 3. Panel B: Data from a representative participant showing the effect of the dose correction on the PETWALK image using Equation 1 when applied to co-registered images in native subject space. The images were then warped to standard Montreal Neurological Institute (MNI) space Top: PETSTAND, middle – uncorrected PETWALK, bottom – corrected PETWALK. The color bar shows image intensity across the three images. Image courtesy of NeuroImage.
The first task involved patients standing upright for 15 minutes in five-minute blocks, with one minute of rest (sitting) in between each block. After this (30 minutes after the F-18 FDG injection), patients underwent a 16-minute PET scan in resting supine positions.
Following a short break, participants received a second F-18 FDG injection and then completed a 15-minute standardized walking task around an 8-meter track, followed by a second 16-minute scan (also 30 minutes post FDG injection).
Finally, during image processing, the researchers applied what they described as “a bespoke dose correction” method to discern independent neural functions related to walking compared to standing. In brief, they removed residual F-18 signals of the first scan (standing) from the second scan (walking) and compared the images.
“Our results with a dose correction and scaling to the global mean showed that walking, compared to standing, increased glucose consumption in the cuneus, the temporal gyrus, and the orbital frontal cortex,” the group wrote.
Ultimately, a detailed understanding of the neural networks involved in the control of gait is still emerging and this has limited the development of interventions to tackle mobility loss and its consequences, the researchers wrote.
The significance of this study is that it establishes the feasibility and tolerability of a new method capable of capturing neural activations related to actual walking, they wrote.
“Our paradigm could be used to explore pathological alterations in various gait disorders,” the group concluded.
The full study is available here.