PET/CT imaging with a new gallium-68-based prostate cancer radiotracer shows promise for detecting recurrent metastatic disease, according to a study published February 20 in the Journal of Nuclear Medicine.
The finding is from a phase I/II clinical trial in the U.S. and supports further development of the imaging agent, noted lead author Liza Lindenberg, MD, of the National Cancer Institute (NCI) in Bethesda, MD, and colleagues.
“Overall, more participants with potential tumor lesions and more potential lesions were detected with Ga-68 PSMA-R2 PET/CT than with conventional imaging,” the group wrote.
To date, several prostate-specific membrane antigen (PSMA) PET radiotracers have been approved in the U.S. for imaging PSMA-positive lesions in patients with newly diagnosed or recurrent prostate cancer, yet the biodistribution of these tracers can vary, the authors explained. Imaging agents with low uptake in at-risk organs and higher tumor uptake are needed, they noted.
PROfind was a phase I/II first-in-human study sponsored by Novartis and conducted at five sites in the U.S. to evaluate the safety and tolerability of gallium-68 (Ga-68) PSMA-R2, as well as its potential for identifying lesions. The radiotracer was developed in 2019 for use in theranostics, the authors noted.
In the trial, 30 patients with recurrent disease or metastatic prostate cancer underwent baseline conventional imaging (CT, MRI, or bone scan) and whole-body Ga-68 PSMA-R2 PET/CT between 20 minutes and four hours after injection. All patients had undergone either radical prostatectomy or radiation therapy.
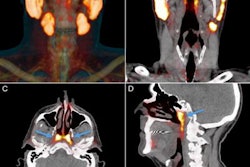
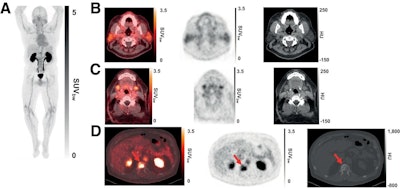 (A) Representative maximum-intensity projection and selected PET/CT images (fused, PET and CT only) of patient with metastatic prostate cancer at 1 hour after injection. (B and C) Low uptake of Ga-68 PSMA-R2 in salivary and submandibular glands is also demonstrated. (D) Images show heterogeneous uptake of Ga-68 PSMA-R2 with varying degrees of intensity in multiple spine bone lesions (vertebral sclerotic lesion with intense PSMA-R2 expression at its periphery indicated with red arrow). HU = Houndsfield unit; SUVbw = body-weight SUV. Images and caption courtesy of the Journal of Nuclear Medicine.
(A) Representative maximum-intensity projection and selected PET/CT images (fused, PET and CT only) of patient with metastatic prostate cancer at 1 hour after injection. (B and C) Low uptake of Ga-68 PSMA-R2 in salivary and submandibular glands is also demonstrated. (D) Images show heterogeneous uptake of Ga-68 PSMA-R2 with varying degrees of intensity in multiple spine bone lesions (vertebral sclerotic lesion with intense PSMA-R2 expression at its periphery indicated with red arrow). HU = Houndsfield unit; SUVbw = body-weight SUV. Images and caption courtesy of the Journal of Nuclear Medicine.
According to the results, 13 adverse events were reported, yet all were mild or moderate and deemed not related to the radiotracer, the group reported. In addition, Ga-68 PSMA-R2 PET/CT detected 85 lesions in 22 participants one hour after injection and 103 lesions in 22 participants two hours after injection. Conversely, conventional imaging detected 49 lesions in eight participants with metastatic disease and none in participants with recurrent disease, according to the analysis.
Ultimately, metastatic prostate cancer has a poor five-year survival rate (32.3%) compared with localized disease and thus early detection is crucial, the authors wrote. The lesions identified only by PET/CT in patients in this study may represent earlier-stage or smaller lesions not detectable by conventional imaging, they noted.
Moreover, the biodistribution and dosimetry data of the PSMA-R2 ligand also indicate it has potential for use as a therapeutic agent when coupled with beta-emitting radionuclides such as lutetium-177 or alpha-emitting radionuclides such as actinium-225, according to the authors.
“Safety and preliminary imaging performance data support further development of Ga-68 PSMA-R2 as a diagnostic agent in patients with [prostate cancer],” the group concluded.
The full study is available here.