For almost 30 years, cochlear implants have instilled a kind of hearing replacement in deaf people who aren't helped by hearing aids. The implants work by stimulating the auditory nerve electronically, and while the results aren't exactly hearing, they can be dramatically effective. A year after surgery, many patients can understand some speech, identify environmental sounds, even enjoy music.
But the implants are expensive -- around $65,000 in the U.S. if one includes the first year of auditory and speech training -- and they don't work for everyone. As a result, preoperative assessment of the temporal bone with CT and/or MRI has become routine for eliminating candidates for surgery, especially those presenting with labyrinthine ossification or congenital defects of the cochlea, two main exclusionary factors.
Last year a retrospective multicenter study of 525 patients in the U.K. found that high-resolution CT was 94.6% accurate in predicting cochlear ossification, with 100% specificity and 71% sensitivity. Meningitis was the leading cause of cochlear ossification among the subjects (44%), and the authors recommended MRI as a useful addition in post-meningitis patients (Clinical Otolaryngology, February 2001, Vol.25:1, pp. 55-61).
A few researchers are looking beyond the temporal bone to the brain itself. They theorize that brain activity in the auditory cortices and beyond may predict surgical outcome better than simple morphologic evaluations.
At the 2001 Society of Nuclear Medicine meeting in Toronto, researchers from the University of Texas Southwestern Medical Center in Dallas offered a study that explored the neuroanatomical function of auditory cortices in candidates for cochlear implantation.
"The goal of the study was to evaluate patients who are deaf prior to the surgical implantation of cochlear implant," said Dr. Michael Devous, presenting the research he conducted with colleagues Dr. Emily Tobey, Dr. Tom Harris, and Dr. Peter Roland. "And in that context, we were interested in seeing if we can aid particularly in deciding which ear should be implanted in the patients who are going to receive these devices as a curative process."
The group evaluated six cochlear implant candidates and 13 normal controls with regional cerebral blood flow (rCBF) SPECT. Images were acquired during four videotaped presentations designed to stimulate the auditory cortices. Postoperative results were available for three of the patients.
All of the subjects watched a 15-minute videotaped story under four different auditory conditions: monaurally in the right and left ears (aided), binaurally (aided) and finally without any audio signal. Their comprehension of the story was tested after each presentation, Devous said.
The methodology of testing each ear separately was needed to establish normal response, because the two sides don't respond the same way, even in normal subjects, Devous told AuntMinnie.com.
"When a patient is assessed [preoperatively], we can't take left/right ear differences as an indication of what ear to implant unless we take into account normal differences in ear responses," he said.
IVs were inserted before the tapes began so that the subjects would be unaware of the time of radiotracer administration. Five minutes after the presentation began, the researchers administered 20 to 25 mCi of technitium 99m hexamethyl-propyleanamine (HMPAO). The scans were acquired over 20 minutes using triple-headed gamma camera (Prism 3000, Marconi, Cleveland, OH), and the resulting images were normalized and co-registered to Talairach space.
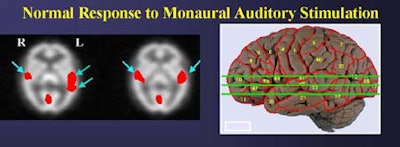
In normal subjects, the right and left-ear monoaural stimulation yielded significant bilateral activation of Brodmann areas 41, 42, 21, 22, and 38. In the monaural (right or left-ear only) studies, the highest activation levels were contralateral to the ear that received the stimulation.
"The distribution is contralateral-dominant, but bilateral once you get into the inferior slices, that is to say, contralateral-dominant in the primary auditory regions of the cortex, and bilaterally asymmetric in the association cortices for this stimulus," he said. "For left-ear presentations the same thing is true, although the contralateral dominance is not as clear for the left-ear presentation as for the right-ear presentation.... In that context we see in normal subjects significant activation of primary and association cortices in Brodmann areas 41, 22, and 38. [Area] 42 is also bilateral, but it is clearly reduced in anatomic extent on the ipsilateral side."
The implant candidates failed to activate these areas bilaterally under monaural presentations. However, bilateral activation of areas 41 and 42 were seen in the binaural tests, Devous said. He followed up with postoperative results from 3 patients.
 |
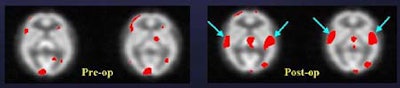 |
"So this implant -- in the ear that did not show dramatic pre-op activation -- nevertheless yielded a dramatic improvement in stimulation of the auditory cortex," Devous said.
However, implantation apparently failed in another subject, a 63-year-old woman who used two hearing aids preoperatively. She demonstrated fairly robust brain stimulation on both sides preoperatively, but a year after receiving a right-ear implant, showed far less stimulation, and also had some hearing loss, Devous said.
"With two hearing aids there was a synergistic effect that gave pretty good stimulation. In this case, apparently the two are not synergistic," he said. "There is some activation, so it's not that the cochlear implant isn't sending out signals; it's not that the auditory nerve isn't sending out signals; it's that whatever the brain is getting is not being recognized as speech. We're still looking at SPECT and trying to understand why poor users are poor users... I suspect we'll see this [result] again."
The final example was a resounding success.
"In this patient [below], even though the two ears appear to be equally deaf based on audiological data, SPECT data shows that the brain is getting more information from the left ear than from the right ear," and the results were validated postoperatively, Devous said.
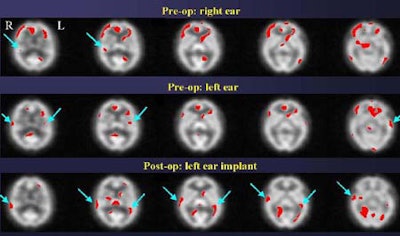 |
Both subjects in which activated auditory cortices are reported preoperatively do so in the context of being unable to report having heard anything. Their auditory comprehension scores are zero after presentation of the stimuli. Thus, the rCBF response is important because it demonstrates that SPECT can predict which ear will have a better postoperative response, Devous said.
"In conclusion, we are able to use a simple SPECT imaging procedure with a fairly staightforward stimulus to map out the distribution of the rCBF response to normal speech perception, and delineate abnormalities that are associated with hearing loss," he said. "We have now 10 patients in this case series of preoperative evaluation, and in most of them we see a distinct difference between the left ear and the right ear, even though our auditory assesssments suggest they are not particularly different, and these differences may predict better outcomes in the ear providing greater stimulus to the auditory cortex."
All patients in the ongoing study are reevaluated annually, Devous said.
By Eric BarnesAuntMinnie.com staff writer
August 16, 2001
Copyright © 2001 AuntMinnie.com