Eli Lilly and its wholly owned subsidiary Avid Radiopharmaceuticals have received a letter from the U.S. Food and Drug Administration (FDA) for their new drug application (NDA) for Amyvid (florbetapir).
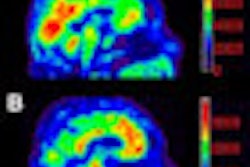
The communiqué for the PET imaging agent, currently under investigation for the detection of beta amyloid plaque in the brains of living patients, focuses primarily on the need to establish a reader training program to help ensure reader accuracy and consistency of interpretations of existing Amyvid scans.
In January, an FDA advisory committee voted unanimously, 16-0, to conditionally recommend approval of Amyvid for detecting beta amyloid plaque deposits that could be precursors to Alzheimer's disease.
The decision stipulated that a training program be made available for readers of PET images acquired with Amyvid to reduce inconsistent interpretations within the patient population intended for the clinical application.
According to Lilly and Avid, the two companies have been working to address the reader training program issues and will continue dialogue with the FDA.