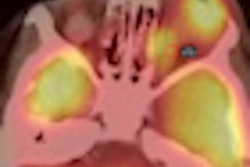
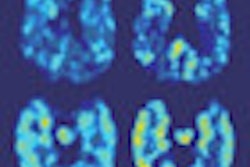
Siemens Healthcare has received U.S. Food and Drug Administration (FDA) clearance for its new Biograph mCT PET/CT scanner.
First introduced at the RSNA 2011 meeting in Chicago, the latest version of Biograph mCT provides measurements of metabolic processes and data quantification, including the assessment of neurological disease, cancerous tissue, and cardiac perfusion, according to the vendor.
It features Siemens OptisoHD detector system, which yields a volumetric resolution of 87 mm3, according to the firm. Siemens has also included time-of-flight (TOF) and HD-PET capability, as well as its Quanti-QC system, which performs daily system normalization, including calibrating and tuning the system.
Siemens Molecular and Anatomical Registration Technologies (SMART) provide attenuation correction and quantitative measurements, as well as auto cardiac registration to align CT and PET heart images automatically. SMART also features a new form of attenuation correction for neurological images that no longer requires CT data, Siemens said.
Advanced quantifiable measurement tools are available with Siemens' syngo applications. A new addition to the syngo.via oncology engine, SUVpeak provides quantitative assessments of hot spots. A new neurology tool, syngo.PET Neuro DB Comparison, automatically registers brain data to an FDG-PET database of normal scans to help assess neurological disorders, Siemens said.
The new system is expected to begin shipping in May.