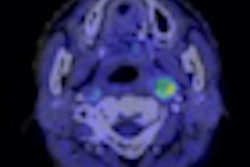
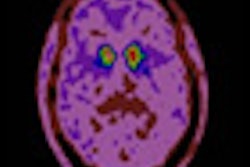
Imaging with PET/CT and the radiopharmaceutical fluorine-18 dihydroxyphenylalanine (F-18 DOPA) can significantly alter treatment management plans for patients with brain tumors, according to a study in the March issue of the Journal of Nuclear Medicine.
A study from the University of California, Los Angeles (UCLA) found that use of F-18 DOPA-PET/CT changed the intended management plan for 41% of patients with brain tumors.
Referring physicians were given a survey prior to performing PET/CT scans with F-18 DOPA on patients with known or suspected brain tumors. The prescan surveys asked about indication, tumor histology or grade, level of suspicion for tumor recurrence, and planned management.
After the PET/CT scans, the referring physicians were asked to categorize PET findings, level of suspicion for primary or recurrent brain tumors, and intended management changes. Six months following the scans, the physicians responded to a third survey detailing disease recurrence and patient survival.
Of the 58 cases included in the survey, clinical suspicion for recurrence increased in 33%, remained unchanged in 50%, and decreased in 17% after adding the PET/CT findings to other diagnostic data.
As a result, there were recommended changes in management, including from a watch-and-wait strategy to chemotherapy (25%), and from chemotherapy to watch-and-wait (17%). In all, 75% of the recommended changes were implemented.
F-18 DOPA-PET/CT imaging is "highly accurate for detecting brain tumors, and in our research we've shown that this imaging modality has a significant impact on patient management," noted study co-author Dr. Johannes Czernin, from UCLA's department of molecular and medical pharmacology.
In the future, he added, the researchers plan to initiate multicenter trials to determine whether these management changes affect patient outcome.