Bayer Pharma is selling its portfolio of molecular imaging agents, including a PET tracer designed to detect early signs of Alzheimer's disease, to Indian healthcare conglomerate Piramal Healthcare.
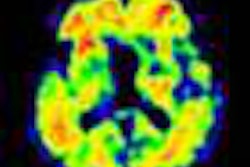
Bayer's portfolio includes rights to florbetaben, which is in the final stages of phase III clinical trials. Florbetaben is a PET tracer for detecting beta-amyloid plaque deposition in the brain, which is the pathological hallmark of Alzheimer's disease. Detection of beta-amyloid deposits is expected to result in earlier diagnosis and more specific treatment of Alzheimer's disease.
News reports published in September 2011 indicated that Bayer was planning to sell the business. In an April 15 press release, Bayer said it was divesting the division to "free up resources to concentrate and accelerate the development of our exciting late-stage pipeline."
The first phase III trial results of florbetaben will be presented on April 25 at the American Academy of Neurology (AAN) annual meeting in New Orleans by the study's coordinating investigator, neurologist Dr. Marwan Sabbagh, medical director of the Banner Sun Health Research Institute in Sun City, AZ.
The phase III trial showed that PET imaging with florbetaben reliably detects beta amyloid in the brain during life with great accuracy, and thus shows value as a potential tool to help diagnose and assess Alzheimer's disease.
Piramal estimates that the emerging new class of PET imaging agents for Alzheimer's disease has a global market potential of up to $1.5 billion, and the company is establishing a dedicated global commercial team for florbetaben. Core members of Bayer's R&D team working on the portfolio will join Piramal Imaging in Mumbai, which will carry forward the development of florbetaben and take it through regulatory approval processes worldwide.
Piramal plans to file for regulatory approvals in 2012.