The combination of 1.5-tesla MRI, FDG-PET, and cerebrospinal fluid (CSF) data when added to routine clinical tests significantly increases the ability to predict whether people with mild cognitive impairment will progress to Alzheimer's disease, according to a study published online December 11 in Radiology.
Researchers from Duke University Medical Center noted that the misdiagnosis rate in the very early stages of Alzheimer's can be as great at 41.3%, given that there are more than 100 conditions that can mimic the disease. By combining MRI, FDG-PET, and CSF biomarkers with clinical tests, they were able to reduce the frequency of misdiagnoses to 28.4%.
The group also found that of the three techniques, FDG-PET was the only one that significantly improved the predictive value of factors such as a person's age, education, and apolipoprotein E (APOE) genotype, which has been identified as a genetic risk factor for Alzheimer's.
The study was led by Dr. Jennifer L. Shaffer from Duke University Medical Center's department of radiology (Radiology, December 11, 2012).
First-of-its-kind study
According to Shaffer and colleagues, this study is the first time the three diagnostic tests have been used together to help predict the progression of Alzheimer's. In previous research, both structural MRI and FDG-PET have proved effective in showing atrophy in the brains of patients with mild cognitive impairment and Alzheimer's, especially in regions that are associated with memory function.
Despite these findings, the modalities "have not yet been proved accurate enough for prognostic use in the routine clinical setting or clinical drug trials," the authors wrote. "It is possible, however, that MRI and FDG-PET are complementary and yield additional information when interpreted jointly."
In this retrospective study, the researchers reviewed the Alzheimer's Disease Neuroimaging Initiative (ADNI) database to identify subjects with mild cognitive impairment who had baseline clinical examinations.
ADNI was launched in 2003 to collect a wide range of data on 200 healthy elderly control subjects, 400 people with mild cognitive impairment, and 200 individuals with Alzheimer's disease. Subjects were recruited from more than 50 sites across the U.S. and Canada.
The inclusion criteria included having 1.5-tesla MRI, FDG-PET, and CSF marker results, as well as information on APOE status and results from at least one follow-up clinical examination, as of October 2010.
Shaffer and colleagues identified 97 subjects with mild cognitive impairment who had a mean age of 75.1 years (± 7.2 years). Of the study cohort, 34 individuals (35%) had a family history of Alzheimer's, while 53 (55%) were positive for the ε4 allele of APOE. The mean follow-up for all subjects was 31.5 months (± 10 months).
To evaluate the images, the researchers extracted whole-brain gray-matter data from the MRI results and coregistered FDG-PET images to the subjects' corresponding MRI studies.
Of the 97 subjects, 43 (44%) progressed to Alzheimer's during follow-up, while 54 (56%) did not develop Alzheimer's. Individuals who progressed to Alzheimer's tended to have longer follow-up times by approximately four months. The average time from the initial screening visit to conversion was 20.7 months (± 9.8 months).
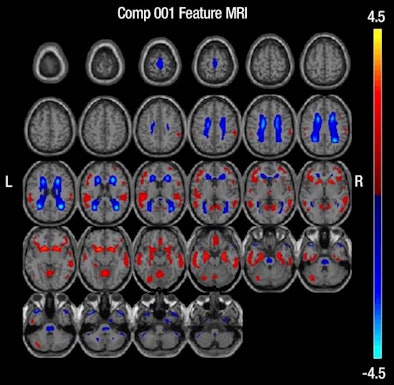 |
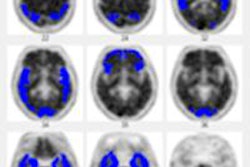
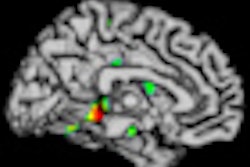
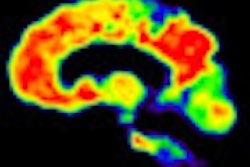
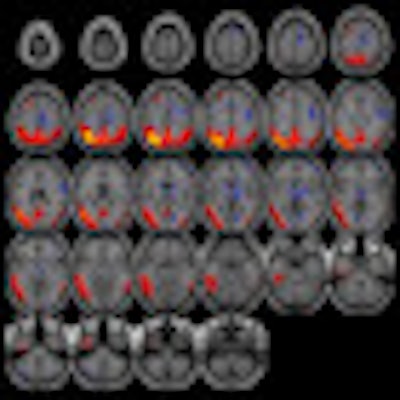
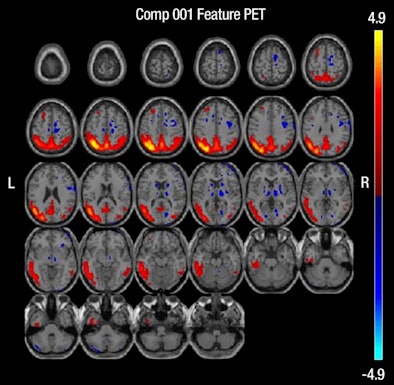
| MR images above highlight in red the bilateral medial temporal lobes, inferior and lateral temporal lobes, and anterior and inferior frontal lobes, consistent with atrophy in brain regions of people who develop Alzheimer's. In blue are negative signals in the periventricular white matter, consistent with higher levels of white-matter disease in those same patients. Below are FDG-PET images in which red areas show the temporoparietal lobes (right greater than left) and the posterior cingulate region, consistent with hypometabolism in the brain regions of patients who progress to Alzheimer's. Images courtesy of Radiology. |
 |
Routine clinical tests alone (age, education, APOE4 status, and cognitive tests) were the least influential in predicting which subjects would progress to Alzheimer's, with a misdiagnosis rate of 41.3%. The combination of MRI, FDG-PET, and CSF proteins with routine clinical tests proved to be the best predictive model, reducing the misdiagnosis rate to 28.4%.
When the researchers evaluated the imaging modalities and biomarkers separately, FDG-PET had the greatest effect on predictive value. The misdiagnosis rate for FDG-PET was 27.2%, compared with 39.2% for MRI and 39.6% for CSF.
Why FDG-PET provided greater accuracy or sensitivity than the other techniques for patients with mild cognitive impairment is unknown.
"There remains lack of consensus on the exact timeline of evolution of FDG-PET deficits in relation to CSF biomarker changes, although there is growing consensus that metabolic deficits are greater in magnitude than volumetric changes earlier in the disease," the authors wrote.
As for the clinical benefit of the findings, study co-author Dr. Jeffrey Petrella, associate professor of radiology and director of Duke's Alzheimer's Disease Imaging Research Laboratory, said that because new treatments are likely to be most effective at the earliest stages of Alzheimer's, there is an urgency to develop sensitive markers that facilitate the detection and monitoring of early brain changes in at-risk individuals.
Additional research should be conducted to evaluate which biomarkers would be the most effective in clinical practice, in addition to examining cost-effectiveness, the authors recommended.
|
Study disclosures Funding for the study was provided in part by ADNI, the National Institutes of Health, the National Science Foundation, and the Dana Foundation. |