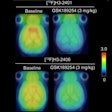
Piramal Imaging said that the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have accepted the company's applications for review of its florbetaben investigational PET amyloid imaging agent.
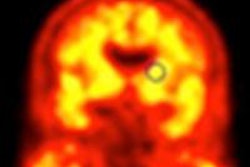
A new drug application was submitted to the FDA and a marketing authorization application to the EMA to use florbetaben for the visual detection of beta amyloid in the brains of adults with cognitive impairment who are being evaluated for Alzheimer's disease and other causes of cognitive decline.
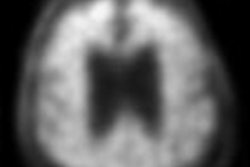
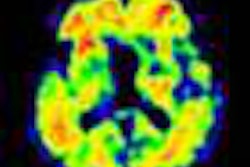
The applications for florbetaben are based on the results of a clinical program that included a multicenter phase III trial to compare in vivo PET imaging of the brain with florbetaben and postmortem analyses of brain tissue.
The study was performed to confirm that florbetaben binds to beta amyloid in the brain at the regional level, and that the radiopharmaceutical is diagnostically useful to exclude Alzheimer's disease.