Siemens Healthcare has garnered U.S. Food and Drug Administration (FDA) 510(k) clearance for its Symbia Intevo SPECT/CT system.
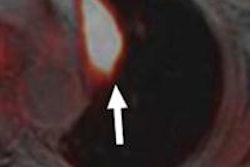
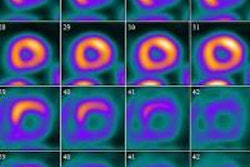
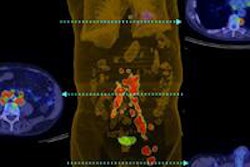
Intevo, the first member of Siemens' xSPECT product family, integrates data from both SPECT and CT modalities and generates high-resolution as well as quantitative SPECT images, according to the vendor.
Siemens has also included its CARE Dose4D technique, which can reduce patient CT radiation dose by up to 68%, while the system's Autoform collimator captures up to 26% more photon counts from radiotracer activity, according to the vendor.
Siemens first unveiled Intevo at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) meeting in June.