Imaging agent developer ImaginAb is touting interim results from a phase II clinical trial of its IAB2M imaging agent for managing prostate cancer.
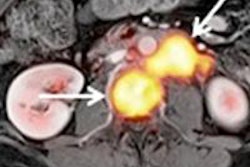
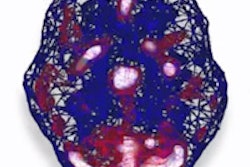
In preliminary data presented at the 2015 World Molecular Imaging Congress (WMIC) in Honolulu, IAB2M outperformed ProstaScint and conventional imaging technologies such as CT, MRI, and bone scans for detecting prostate cancer, ImaginAb said. What's more, the agent accurately detected metastatic disease in normal-sized lymph nodes, according to the vendor.
The study results include the first nine of 20 patients participating in an ongoing, single-center, phase II trial performed at the University of California, Los Angeles (UCLA) Jonsson Comprehensive Cancer Center. IAB2M, which was given intravenously to patients prior to a whole-body PET scan, detected disease in the lymph nodes of three of six patients, which were later confirmed as positive for metastasis at surgery, ImaginAb said. ProstaScint and conventional imaging did not detect metastasis in the lymph nodes in any of those subjects.