The U.S. Drug Enforcement Administration (DEA) has removed a compound found in GE Healthcare's DaTscan SPECT radiopharmaceutical from the schedules of the Controlled Substances Act, which means the imaging agent will be more widely available to the medical community.
The DEA removed ioflupane iodine-123 from the schedule II controlled substance list, which had restricted its use to only those healthcare professionals and imaging facilities registered with the DEA and able to comply with all of the requirements for the appropriate prescribing, storage, use, and disposal of schedule II controlled substances. Descheduling has removed these roadblocks, making it easier for imaging specialists to order DaTscan and administer it in their facilities, GE said.
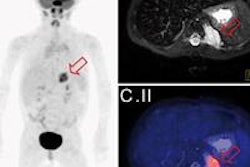
DaTscan is used to assess the integrity of the striatal dopaminergic system via the dopamine transporter to help physicians determine whether patients have essential tremor or a parkinsonian syndrome movement disorder such as Parkinson's disease.
The radioactive drug is injected into the bloodstream to help image areas of the brain using SPECT.