A new dedicated breast PET device was put through its paces by European researchers in an article in the June issue of the American Journal of Roentgenology. While the system excelled at identifying lesions smaller than 1 cm, its limited field-of-view could be an issue, they found.
The group from Spain and the Netherlands found that the Mammi-PET system from OncoVision detected 98.6% of primary breast cancer lesions within its field-of-view, including smaller lesions missed by PET/CT. But the Mammi-PET unit missed a number of lesions that were outside of its scanning range and found by PET/CT -- a potential limitation (AJR, June 2016, Vol. 206:6, pp. 1307-1314).
The exam table "has to be adjusted to obtain a more optimal scanning range while the benefits of the new device are preserved," wrote the authors led by Dr. Suzana Teixeira from the Netherlands Cancer Institute in Amsterdam. "This technical issue needs to be optimized to enable visualization of the most dorsal breast lesions."
The breast PET alternative
Breast imaging specialists have pursued a variety of modalities to serve as an adjunct to mammography and for follow-up imaging. For example, PET/CT is currently recommended for distant metastasis screening in stage II-III breast cancer. Researchers have also pursued the imaging of women in the prone position, following the success of the position in breast MRI.
However, previous research has found that PET/CT's clinical value is "limited because its sensitivity decreases markedly for tumors smaller than 1 cm," Teixeira and colleagues noted. Dedicated molecular breast imaging systems such as breast PET and breast-specific gamma imaging have also been introduced, but these also have disadvantages, such as radiation dose and reduced ability to detect lesions smaller than 1 cm.
To overcome these obstacles, European researchers developed Mammi-PET, a high-resolution, full-ring PET device designed to create 3D images of the breast with no need for compression. The patient lies facedown with her breast hanging through an opening in the exam table. The system's field-of-view is approximately 19 cm.
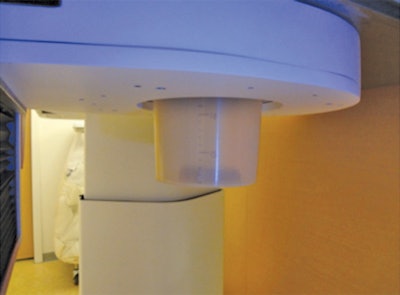 With Mammi-PET, the patient hangs her breast through an opening in the exam table and into a sleeve to measure the length of the breast. All images courtesy of AJR.
With Mammi-PET, the patient hangs her breast through an opening in the exam table and into a sleeve to measure the length of the breast. All images courtesy of AJR.The original Mammi-PET prototype was developed in 2010, and subsequent research at the Mayo Clinic in Rochester, MN, found that the system had spatial resolution of around 1.6 mm, versus 5 mm to 6 mm for whole-body PET. It also requires only 2.5 mCi to 4 mCi of radiopharmaceutical, compared with 10 mCi to 15 mCi for whole-body PET.
OncoVision, a Valencia, Spain-based technology start-up created in 2003 by the University of Valencia and the Corpuscular Physics Institute, provided the Mammi-PET system for the current study as part of an unrestricted institutional grant for clinical validation by the participating imaging centers. The centers maintained complete control of the data and analyses, according to the authors.
Imaging protocols
The prospective study enrolled 230 women with a mean age of 52 years (range: 24-82 years) at two participating hospitals between March 2011 and March 2014. The women had one or more histologically confirmed primary breast cancer lesions, or index lesions. Four patients had cancer in both breasts, bringing the total number of index lesions to 234; the lesions ranged in diameter from 5 mm to 170 mm. All patients had previously undergone mammography, ultrasound with ultrasound-guided biopsy, and MRI to characterize the tumors.
Both imaging facilities used a standard whole-body PET/CT protocol, with patients fasting for six hours before administration of FDG. A dose of 180 MBq to 240 MBq of FDG was injected intravenously based on body mass index. After a resting period of 60 minutes (± 10 minutes), PET/CT scans (Gemini TF, Philips Healthcare) were performed from the skull base to the groin with the patient in a supine position to screen for distant metastases.
Approximately 110 minutes after injection, the Mammi-PET scan was performed with the patient positioned facedown and her breast containing the tumor hanging freely in the opening of the device. The 234 index lesions scanned with Mammi-PET included 11 lesions smaller than 10 mm.
Lesion detection
Mammi-PET's performance varied mostly based on where lesions occurred in the breast: The system found 95% of index lesions that were 1.5 cm or farther from the pectoral muscle, but missed 80% of index lesions that were 8 mm or closer to the muscle, outside of the system's scanning range.
Mammi-PET missed 26 index lesions in total: 23 (88%) were outside of the device's scanning range and 13 (50%) were hidden by the pectoral muscle.
The device achieved an overall sensitivity of 89% (208 of 234 lesions detected). Sensitivity increased to 99% (208 of 211 lesions detected) when the researchers omitted missed tumors outside of the scanning range.
Among the 230 women, 206 underwent both whole-body PET/CT and Mammi-PET scans. In this group, Mammi-PET again missed 23 index lesions, with 20 lesions (87%) outside of the device's scanning range. PET/CT missed 20 index lesions; interestingly, Mammi-PET discovered 16 of the 20 index lesions missed by PET/CT.
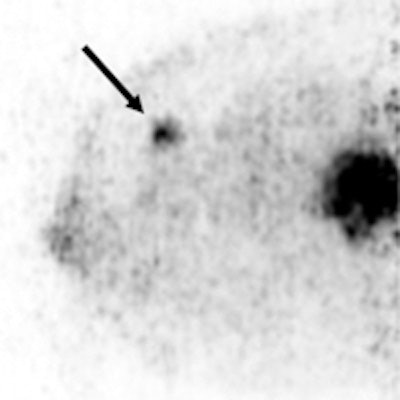
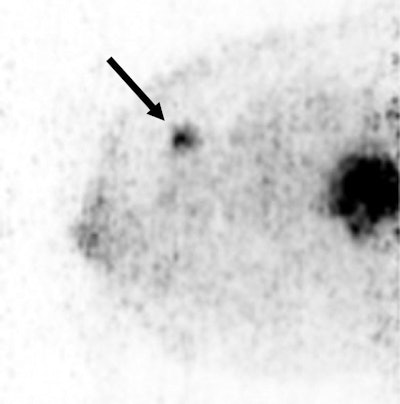 A 36-year-old woman with a large triple-negative ductal type carcinoma has two FDG-avid lesions on Mammi-PET. Arrow indicates smaller lesion.
A 36-year-old woman with a large triple-negative ductal type carcinoma has two FDG-avid lesions on Mammi-PET. Arrow indicates smaller lesion.Based on those results, Mammi-PET again had a sensitivity of 89%, compared with 91% for PET/CT. The difference in performance of the two techniques was not statistically significant (p = 0.61), the authors noted.
They also found that Mammi-PET detected nine of 11 index lesions smaller than 1 cm in diameter that were within its scanning range. In comparison, PET/CT found only one of eight index lesions smaller than 1 cm.
Study limitations
Teixeira and colleagues acknowledged several limitations of the study, including the concern that some lesions were missed due to Mammi-PET's restricted scanning range. They noted that a previous version of the system was able to detect lesions starting at 3 mm from the pectoral muscle, rather than the 8 mm with the current system, but the older system was redesigned to provide more stability and support for the patient.
"The examination table has to be adjusted to obtain a more optimal scanning range while the benefits of the new device are preserved," they wrote.
Still, given the similar sensitivity between Mammi-PET and PET/CT for detecting primary tumors, breast cancer patients could benefit from Mammi-PET's shorter scan times and higher sensitivity for smaller lesions, according to the authors. Also, other dedicated chest imaging techniques have trouble with tumors closer than 2 cm to the chest wall.
"Considering that the sensitivity for the primary tumor was similar to that of PET/CT, we suggest as a future perspective for Mammi-PET its clinical use in patients with TXN0 breast cancer," the authors wrote.
They also encouraged the further development of a Mammi-PET device with a module for FDG-guided biopsy, which is being developed with funding from the European Commission.
"Such a device would enable tissue sampling of the most FDG-avid parts of the primary tumor for histopathologic analysis," they wrote.