CHICAGO - An extension of its tablet-controlled Somatom go CT concept into new price points, a new flagship mammography system, and a new product launch for MRI scans of the knee are among the highlights in the RSNA 2017 booth of Siemens Healthineers.
CT
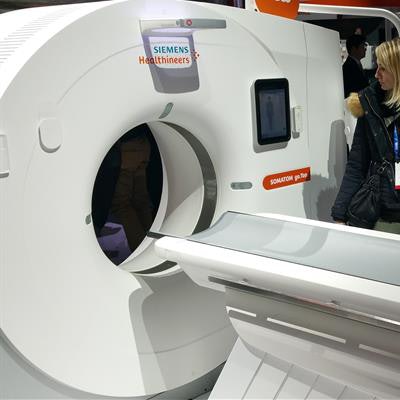
In CT, the biggest news is that Siemens is extending the Somatom go tablet-based CT workflow concept introduced at RSNA 2016 with the launch of two new scanners, Somatom go.All and Somatom go.Top.
The new systems enable users at higher-end price points to take advantage of the go concept, in which all scanner functions can be controlled using a tablet computer. This gives technologists more flexibility and enables them to stay closer to patients.
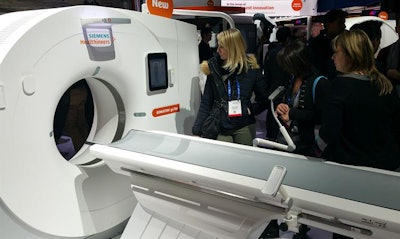 Somatom go.Top is one of two new CT scanners being launched at RSNA 2017.
Somatom go.Top is one of two new CT scanners being launched at RSNA 2017.Somatom go.All and Somatom go.Top are designed for more advanced applications, such as cardiac scanning and CT-guided interventional procedures. Go.All is a 64-slice scanner and Go.Top is a 128-slice system; both feature a gantry rotation speed of 0.33 sec.
Siemens has also introduced several new apps on the tablet to help control various scanner functions, including Guide and Go for interventional procedures, Recon and Go for automated image reconstruction, and Check and Go to confirm organ coverage. Somatom go.Top also includes a twin-beam dual-energy application as an option.
Somatom go.All and go.Top include a 6 mega-heat-unit (MHU) Athlon tube that supports energy settings in 10-kV steps at high mA. The table load of the scanners is 227 kg, with a heavy-load option that brings the weight capacity up to 300 kg. A tin filter is also available for low-dose scanning.
The new go scanners will be available in the second quarter of 2018, depending on regulatory clearances. Siemens plans to make the new apps and enhancements on the tablet computers available to the installed base sites with existing go scanners.
At RSNA 2017, Siemens is also introducing new features and enhancements on other CT scanners in its portfolio. Siemens' flagship dual-source CT scanner, Somatom Force, has a new look and feel with new gantry covers, as well as more automated features such as automated cardiac scanning from the console. The system also has two new Vectron x-ray tubes with a focal spot of 0.3 mm to 0.5 mm.
The vendor's premium single-source scanner, Somatom Edge, has been upgraded to the Edge Plus model, with a Straton MX Sigma tube with a high mA range, Stellar Infinity detectors, tin filters, and twin-beam dual-energy scanning. The system has organ coverage of 230 mm per second with a 0.28-sec gantry rotation time and 78-cm bore, which is useful for scanning obese patients.
The company is also demonstrating a 3D camera, called Fully Assisting Scanner Technologies (FAST), designed to assist with patient positioning. The FAST 3D camera is mounted on the ceiling directly above the patient table, and it acquires a 2D image of the patient that also includes 3D data such as anatomical landmarks and information about the patient's size, height, and weight. It can also notify users if a patient is positioned incorrectly. The camera will be available on the new Edge Plus, Somatom Drive, and new Somatom Force scanners, and an upgrade will be made available to the installed base of Force and Drive customers with dual-source systems.
MRI
In the realm of MRI, Siemens debuted its new GoKnee3D MRI application designed to reduce the time for diagnostic knee exams from the standard 20 minutes to approximately 10 minutes. High-resolution isotropic 3D images subsequently allow for evaluation in all planes, including double oblique and curved planar.
GoKnee3D's volume acquisition is based on a Caipirinha SPACE (sampling perfection with application-optimized contrast with different flip-angle evolution) protocol, which allows enhanced scan speeds and optimal image reconstruction for better signal quality. It is also supported by high-channel knee coils and an automated field-of-view adaptation based on machine learning and artificial intelligence.
GoKnee3D is available as an upgrade for the Magnetom Skyra 3-tesla and Magnetom Aera 1.5-tesla MRI scanners, with an eventual rollout planned for other MRI devices in the company's portfolio.
Siemens is also touting the recent clearance from the U.S. Food and Drug Administration (FDA) of its Magnetom Terra 7-tesla scanner. The Mayo Clinic in Rochester, MN, is the first facility in North America to have access to the device. Magnetom Terra's dual-mode feature allows users to switch between an investigational research mode and the 510(k)-cleared clinical mode for imaging, thus maintaining research data and clinical images on separate databases.
Women's imaging
In women's imaging, Siemens is launching Mammomat Revelation, the company's new premium digital mammography platform that supports digital breast tomosynthesis with a wide 50° arc of the system's x-ray tube head.
 Mammomat Revelation features a wide 50° scanning range.
Mammomat Revelation features a wide 50° scanning range.Siemens believes that Revelation's wide-angle acquisition confers a number of benefits, such as higher depth resolution, which produces higher-quality 3D images that make it possible to detect subtle lesions even earlier. Clinicians can also perform biopsies using an HD breast biopsy device that enables one-click targeting of suspicious areas with an accuracy of ± 1 mm.
Revelation also includes the company's InSpect integrated breast biopsy tool, which means that tissue specimens don't have to be taken to a second system for analysis. This reduces breast compression time for patients, as biopsy samples can be imaged and visualized at the technologist workstation within 20 seconds without radiation exposure to the patient.
Revelation also provides automated breast density measurements at the point of the exam; this helps generate personalized risk stratification while also allowing the facility to provide supplemental imaging, giving patients results faster and minimizing uncertainty and stress.
The system also supports functional breast imaging with the company's titanium filter contrast-enhanced mammography mode, which enables users to acquire functional information with morphological detail -- a task typically reserved for a modality like MRI.
Finally, Siemens addressed patient comfort with what the company calls Personalized Soft Compression, in which the breast compression process is softened and the compression force is automatically and individually adjusted.
Revelation has received the CE Mark for marketing in Europe; it is awaiting 510(k) clearance from the FDA.
Molecular imaging
Siemens' molecular imaging lineup for RSNA 2017 has its sights set on 2018. On the horizon is Siemens' Biograph Vision next-generation PET/CT scanner designed for neurology, oncology, and cardiology. If and when the hybrid system is cleared in the U.S. or Europe, it will feature the first detector with smaller (3.2 mm) lutetium oxyorthosilicate (LSO) crystals to better visualize smaller lesions, among other applications. Extended coverage of the silicon photomultiplier sensors is designed for enhanced time-of-flight to improve contrast and signal-to-noise ratio.
The goal with Biograph Vision is also to reduce scan time and injected dose to improve throughput, lower patient exposure to radiation, and reduce tracer cost. It also has an automatic self-calibrating feature, according to the company.
Two other products under development and not yet commercially available are the CardioFreeze and OncoFreeze motion management features. CardioFreeze divides images into multiple gates and then reconstructs them to provide physicians with information on cardiovascular performance to image the human heart. OncoFreeze is designed to show similar sharp delineation of liver metastases with enhanced overall image quality, count statistics, and low noise levels along with a high lesion-to-background ratio.
In addition, Siemens is developing its new Biograph Horizon Flow PET/CT scanner with FlowMotion technology to advance standardized indication-based protocols, adjusted to a patient's anatomy in one single scan.
Interventional and x-ray
In interventional imaging, Siemens is launching nexaris, a new concept that combines multiple imaging modalities into image-guided therapy suites.
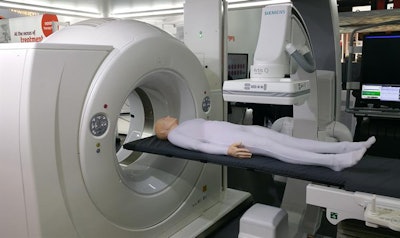 Nexaris is Siemens' concept for combining imaging modalities in the interventional suite.
Nexaris is Siemens' concept for combining imaging modalities in the interventional suite.For example, nexaris Angio-CT combines a Somatom CT scanner on rails in the same room as an Artis angiography system; interventional radiologists can perform a procedure under angiography guidance; slide the patient into the CT scanner to confirm the positioning of a device, for example; and then resume the procedure. The company's Instant Fusion, which is pending FDA clearance, co-registers CT and angiography images automatically, dispensing with manual image fusion, according to the company.
The CT scanner used in a nexaris room can slide up to 39 ft, giving users additional flexibility in siting the system. The scanner could be used for routine diagnostic work outside the interventional suite, for example, and then moved through doors into the suite for interventional work when needed. The nexaris Angio-CT concept also supports the integration of ultrasound, with Siemens' Acuson Freestyle wireless scanner; ultrasound images can be displayed alongside images from nexaris Angio-CT.
Nexaris can also be used to combine the company's Artis pheno angiography system, Magnetom Aera 1.5-tesla MRI scanner, and Magnetom Skyra 3-tesla MRI unit into a multiroom configuration, according to the company. Siemens has partnered with Swedish healthcare firm Getinge on the Pilot system, which enables patients to be transferred to an MRI scanner on a Maquet Magnus table that is combined with a Combi dockable table; patients are laid on a transfer board made of Kevlar and can be transferred to the MRI scanner and slid into the magnet bore. Siemens is awaiting FDA clearance to market the Magnus table with its products.
Ultrasound
In ultrasound, Siemens is highlighting recent new launches and upgrades across its product line -- 90% of the company's products are new or upgraded, according to the vendor. Siemens has added new capabilities in elastography and contrast imaging, as well as image fusion.




















