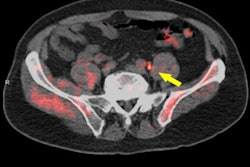
Molecular imaging firm Blue Earth Diagnostics is touting results from an investigational clinical trial with its Axumin F-18 fluciclovine PET/CT radiopharmaceutical.
Blue Earth said PET/CT with Axumin revealed one or more sites of disease recurrence in men with biochemically recurrent prostate cancer. Also, based on the pattern of recurrence, the intervention resulted in a change in management in 59% of the patients.
The results were from the LOCATE trial, a prospective, multicenter, open-label study conducted at 15 sites in the U.S. The trial measured the percentage of men with suspected biochemical recurrence of prostate cancer following initial prior therapy whose treatment plan was changed following an F-18 fluciclovine PET/CT scan.
The researchers recorded a patient's intended treatment plan before Axumin imaging and then how the plan was altered after patients and their physicians had reviewed the results of the scan. Results of the trial indicated that 59% (126/213) of patients had their clinical management changed when results of Axumin imaging were added to the diagnostic workup. Of those changes, 78% (98/126) were classified as "major" (i.e., a change in treatment modality).