Monitoring response during lutetium-177 (Lu-177) prostate-specific membrane antigen (PSMA) radioligand therapy improves outcomes, according to research presented at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) meeting in Chicago.
Why? Because dosing intervals can be tailored, a team led by Andrew Nguyen, MBBS, of St. Vincent's Hospital in Sydney, Australia, found, noting that "early stratification with Lu-177 SPECT/CT allowed men responding to treatment to take a 'treatment holiday' and allowed those not responding the option to switch to another treatment."
"Currently, a standardized dosing interval is used for [Lu-177] PSMA treatment," Nguyen said in a statement released by the society June 27. "However, monitoring early-response biomarkers to adjust treatment intervals may improve patient outcomes."
The U.S. Food and Drug Administration cleared lutetium-177 for prostate indications in 2022. It is used to treat metastatic castration-resistant prostate cancer. Yet how patients respond to this treatment varies, Nguyen and colleagues noted.
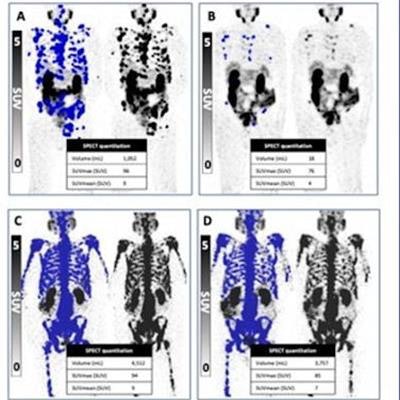
 Outcomes from response groups based on PSA levels and Lu-177 SPECT at six weeks. Image courtesy of Andrew Nguyen, MBBS, and the SNMMI.
Outcomes from response groups based on PSA levels and Lu-177 SPECT at six weeks. Image courtesy of Andrew Nguyen, MBBS, and the SNMMI.The investigators conducted a study to evaluate patients' progression-free survival and overall survival by different Lu-177 dosing intervals. The research included 125 men treated with six weekly doses of Lu-177; all underwent SPECT/CT imaging after each dose. The team analyzed the men's prostate specific antigen (PSA) levels and response to the Lu-177 treatment after the second dose to determine how to proceed.
The men were grouped by the following treatment responses:
- Group 1 (35%) showed a reduction in PSA level and partial response on Lu-177 and were advised to cease treatment until PSA levels increased again.
- Group 2 (34%) showed stable or reduced PSA and stable disease on Lu-177 SPECT imaging; these men continued on their six-week treatment protocol.
- Group 3 (31%) showed a rise in PSA levels and had progressive disease on Lu-177 SPECT imaging; these men were offered the option of trying a different treatment.
PSA levels decreased by more than 50% in 60% of patients, the team noted, and overall, study participants had a median PSA progression-free survival of 6.1 months and a median overall survival of 16.8 months.
| Survival rates by response to Lu-177 treatment for prostate cancer | ||
| Group | Progression-free survival | Overall survival |
| Group 1 | 12.1 months | 19.2 months |
| Group 2 | 6.1 months | 13.2 months |
| Group 3 | 2.6 months | 11.2 months |
"Personalized dosing allowed one-third of the men in this study to have treatment breaks while still achieving the same progression-free and overall survival outcomes they would have if they received continuous treatment," Nguyen said in the SNMMI statement. "It also allowed another one-third of men who had early biomarkers of disease progression the opportunity to try a more effective potential therapy if one was available."