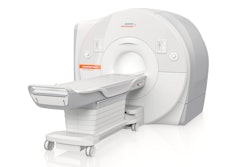
Siemens Healthineers has received U.S. Food and Drug Administration (FDA) clearance for its new Biograph Vision.X PET/CT scanner.
The Biograph Vision.X builds on the performance of the company’s Biograph Vision family of scanners and features an industry-best time of flight (TOF) of 178 picoseconds (ps), according to the company.
The scanner’s Optiso Ultra Dynamic Range (UDR) detector technology contains silicon photomultipliers (SiPMs), which enable the use of small 3.2 mm x 3.2 mm lutetium oxyorthosilicate (LSO) crystal elements. These tiny crystals provide higher spatial resolution than larger crystals, Siemens said. Leveraging these very small LSO crystals, the Biograph Vision.X delivers a 48-mm3 volumetric resolution and a temporal resolution of 178 ps. For these reasons, the scanner can deliver a 20% performance improvement (TOF gain) that can improve patient throughput as well as reduce patient radiation exposure and radiotracer cost, Siemens noted.
 Siemens Healthineer's Biograph Vision.X PET/CT scanner. Image courtesy of Siemens Healthineers.
Siemens Healthineer's Biograph Vision.X PET/CT scanner. Image courtesy of Siemens Healthineers.
In addition, Vision.X includes the firm's AIDAN AI platform, which aims to enhance operational efficiency and accelerate patient workflow. The scanner also includes a 78-cm bore and can fit into any room that houses any other Biograph Vision PET/CT system.
The Biograph Vision.X is available as an in-field upgrade to current users of the Biograph Vision, Siemens said. The company unveiled the scanner as a work in progress at the 2023 Society of Nuclear Medicine and Molecular Imaging annual meeting in June and will showcase Biograph Vision.X at RSNA 2023 next week in Chicago.