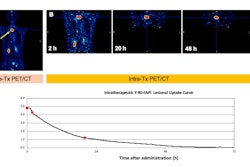
Nuclear medicine firm Curium has submitted a new drug application (NDA) for its lutetium-177 (Lu-177) DOTATATE radiopharmaceutical for treatment of somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumors (GEP-NETS).
The radiopharmaceutical is designed to be injected into patients to deliver Lu-177 radiation directly to tumors. Curium has been developing the formulation over the past several years and has ensured that it avoids infringement of any Orange Book listed patents, it noted.