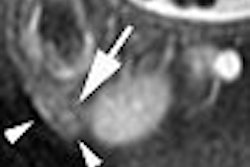
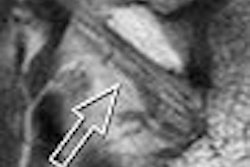
The accumulation of a gadolinium-based formulation in tumors can be used to map pre-existing necrosis and evaluate early tumor response, according to an experimental study done at the Houston-based University of Texas M. D. Anderson Cancer Center.
During radiotherapy and chemotherapy, there is often no decrease in tumor size but there is increased necrosis within the tumor, which can be predictive of improved long-term survival, depending on the type of cancer, explained Chun Li, Ph.D., and colleagues in the International Journal of Radiation Oncology, Biology, Physics (July 1, 2007, Vol. 68:3, pp. 830-838).
Rather than simply measuring the size of necrosis, Li's group focused on how to discriminate between viable tumor and radiation necrosis using an MR contrast agent that "selectively accumulates in necrotic tissue and provides high contrast-to-noise ratio."
For this animal experiment, the researchers used two agents that they created, L-PG-DTPA-Gd and D-PG-DTPA-Gd. L-PG is a synthetic polyamino acid that is degraded by lysosomal enzymes, while D-PG cannot be degraded, they explained. They combined these acids with gadopentetate dimeglumine (Magnevist, Berlex Imaging, Wayne, NJ).
Mice bearing two solid different tumor models -- Colo-205 and OCA-1 tumors -- were injected with the gadolinium-chelated polyglutamic acids. The Colo-205 mice had multiple L-PG DTPA-Gd injections (0.04 mmol Gd/kg) and underwent MRI before each one and after the last injection. The OCA-1 mice also had multiple injections of L-PG DTPA-Gd (0.04 mmol Gd/kg) and D-PG-DTAP-Gd (0.02 mmol Gd/kg). All MR imaging was done on a 4.7-tesla scanner (Bruker BioSpin, Billerica, MA).
MRI data were analyzed with pixel-by-pixel signal intensity (SI) values for the tumors based on the regions of interest (ROIs) on precontrast T1- and T2-weighted images. After the mice were euthanized, the tumor was marked, cut into cranial and caudal portions, and stained with hemotoxylin and eosin (H&E).
The results showed that the central zone of the tumor was significantly enhanced on the T1-weighted images. The stained tumor sample showed areas of necrosis that closely resembled areas of positively enhanced signal on MR. The regions that demonstrated positive enhancement on L-PG-DTPA-Gd MRI were a "bean-shaped" area of coagulation and liquid necrosis in the top left portion of the tumor, the authors explained.
After two days, the necrotic lesions were undetectable on T1-weighted images because they appeared isointense relative to the surrounding normal tissues in treated and untreated tumors, the authors stated.
While success was found with T1-weighted imaging, T2-weighted results were less helpful. "Although the T2-weighted image showed the presence of presumed necrosis in the central zone of the tumor, the distribution of the hyperintense areas was poorly defined and did not match the area of necrosis in the H&E-stained section," they wrote.
The group concluded that both polyanionic contrast agents induced a "strong and persistent" enhancement in sold tumors. The affinity for these agents for necrotic tissue was most likely mediated through macrophages, they added.
"Our data suggest that for these models to work, it is necessary that the contrast agents are in polymeric form, which has prolonged blood half-life … and thus may give sufficient time to interact with migrating or residing macrophages," they explained.
In addition to its potential value for mapping and assessing treatment response, the technique has shown the ability to measure changes in tumor-to-muscle signal ratio, ultimately quantifying the extent of therapy-induced necrosis.
By Shalmali Pal
AuntMinnie.com contributing writer
July 19, 2007
Related Reading
Tumor 'paint' promises better intraoperative visualization of cancer foci, July 17, 2007
CE US sonography makes the grade for measuring tumor vascularity, September 24, 2004
Copyright © 2007 AuntMinnie.com