MR firm Time Medical has received U.S. Food and Drug Administration (FDA) clearance for its hybrid Mona 0.2-tesla MRI and high temperature superconducting (HTS) orthopedic RF coil.
Hybrid Mona MRI is intended for imaging extremities in adults, as well as for head imaging in children, the Metuchen, NJ-based company said.
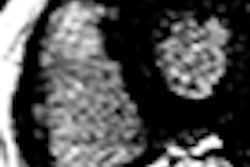
The company touts significantly higher image quality enabled by the HTS orthopedic RF coil over other conventional low-field strength systems using copper coils.
Related Reading
Time Medical to develop breast MRI scanner, October 27, 2009
Time Medical debuts two MR systems, September 28, 2009
Time Medical pursues China venture, April 13, 2009
MR start-up debuts HTS coil, September 18, 2008
Copyright © 2009 AuntMinnie.com


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)




.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)











