The Encompass Group said its Thermoflect blankets should not be used in MRI procedures after the U.S. Food and Drug Administration (FDA) reported an injury to an MRI patient.
The McDonough, GA-based company issued the recall December 26, advising customers that its products should not be used in "MR-conditional or MR-compatible environments." But the blankets are still safe for use in treating hypothermia, a press released noted.
There is no evidence the blankets caused the injury, according to the company, but as a "precautionary measure" Encompass will re-label the product.
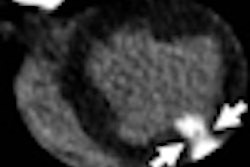
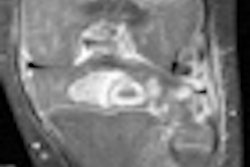
The patient suffered third-degree burns, but company spokeswoman Jea Gackowski noted that the blankets have been used for 17 years in MRI procedures with no prior problems.
Related Reading
FDA warns of pacemaker, MRI issues, December 17, 2009
Rise in MRI accidents highlights need for magnet safety, August 11, 2009
FDA warns of sandbags in MRI suites, April 3, 2009
U.S. FDA warns against wearing skin patch during MRI, March 6, 2009
Joint Commission may survey for MRI safety, March 5, 2009
Copyright © 2009 AuntMinnie.com



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)











