Oncology drug development company Celsion and Philips Healthcare announced the joint resubmission of an investigational new drug/investigational device exemption (IND/IDE) application with the U.S. Food and Drug Administration (FDA) for a phase II clinical study.
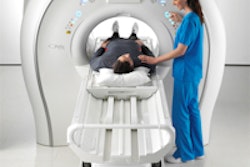
The proposed clinical study would combine Celsion's ThermoDox, a phase III heat-activated liposomal encapsulation of doxorubicin, with Philips' Sonalleve MR-HIFU device, which uses MR-guided high-intensity focused ultrasound for the treatment of prostate cancer metastases to the bone.
The combination treatment would be investigated for pain palliation in patients with painful bone metastasis. Cancer progresses to the bone in a majority of patients with late-stage breast, prostate, or lung cancer, with estimates of between 300,000 to 500,000 cases annually in the U.S.
Philips' MR-HIFU system has the potential to target lesions with acoustic energy, creating sufficient heat to activate ThermoDox and preferentially release high concentrations of doxorubicin in the targeted treatment area, according to the companies.


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)




.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)











