Image-guided therapy developer ViewRay announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its MRI-guided radiation therapy (RT) system.
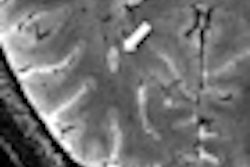
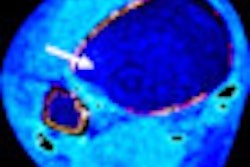
The ViewRay system combines radiotherapy delivery with MRI for the treatment of cancer. The unit offers continuous soft-tissue imaging during treatment so that clinicians can see and record where radiation therapy is being delivered, according to the firm.
ViewRay recently closed the final segment of a $45 million round of venture capital financing intended to help bring the system to market, the company said. The first ViewRay system has already been installed at the Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine.


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)











