Advanced visualization software developer Imaging Biometrics said it's participating in a national network of imaging investigators that are seeking to translate novel MR imaging technologies into practice for monitoring brain cancer treatment response.
Under the five-year, $2.2 million grant from the U.S. National Institutes of Health (NIH), research and laboratory resources will combine with industrial expertise to develop and validate standard and novel imaging biomarkers to monitor treatment response in patients with brain tumors, according to Imaging Biometrics. The company is a subawardee of the NIH grant.
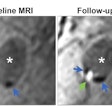
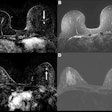
The federal funds will be used to research, develop, and translate into practice both standard and novel perfusion-weighted imaging (PWI) and diffusion-weighted imaging (DWI) biomarkers for clinical trials and standard medical care, the vendor said. While both PWI and DWI have demonstrated promise for treatment response, preliminary studies suggest that combined image maps may present the most complete assessment of treatment response, Imaging Biometrics said.
When included with patented technology from Imaging Biometrics and exclusively licensed technology from other institutions, the result of the NIH grant will be an integrated image analysis platform for use in large-scale, multicenter clinical trials, according to the firm. The company said it also anticipates that the effort may result in an advanced imaging platform that could lead to greater clinical trial efficacy, more rapid drug discovery and commercialization, and improved patient care.



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)




.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)