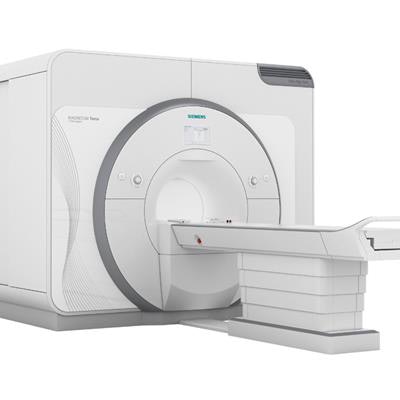
The U.S. Food and Drug Administration (FDA) has cleared Magnetom Terra from Siemens Healthineers, the first 7-tesla MRI scanner cleared for clinical use in the U.S.
The device underwent a premarket 510(k) application process, for which the FDA reviewed the safety of its radiofrequency subsystem through computational modeling, simulations, and experimental validation. In addition, Siemens provided data from a study of 35 healthy patients that compared images acquired using Magnetom Terra with those taken using a 3-tesla unit.
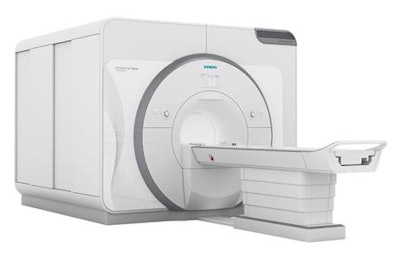 Siemens' 7-tesla Magnetom Terra scanner. Image courtesy of Siemens.
Siemens' 7-tesla Magnetom Terra scanner. Image courtesy of Siemens.Radiologists reviewed the images and confirmed that those acquired by Magnetom Terra were of diagnostic quality and, in some cases, better than those acquired on the 3-tesla device, the FDA said. The scanner is indicated for patients weighing more than 66 lb, with the clearance limited to imaging of the head, arms, and legs, according to the FDA.
Prior to Terra's clearance, the most powerful MRI scanners on the U.S. market were 3-tesla systems, the agency noted.
The clearance is a step forward for MRI technology, according to Robert Ochs, PhD, director of the division of radiological health in the FDA's Center for Devices and Radiological Health.
"The overall image quality of MRI improves with higher magnetic field strength," Ochs said in a statement. "The added field strength allows for better visualization of smaller structures and subtle pathologies that may improve disease diagnosis."
In its own press release, Siemens noted that Magnetom Terra includes a dual-mode feature that allows users to switch between an investigational "research mode" and a 510(k)-cleared "clinical mode" for imaging. The feature keeps research data and clinical images on separate databases, the company said.
Also, Terra sports 64 receive channels, with more than twice the signal-to-noise ratio of 3-tesla MRI scanners when operating in neuro and musculoskeletal clinical applications that have been optimized for 7-tesla imaging. Its gradients, rated at an amplitude of 80 mtesla/m and a slew rate of 200 tesla/m/sec, are suitable not only for diffusion MRI and functional MRI (fMRI), but also for Siemens' Simultaneous Multi-Slice (SMS) application to accelerate advanced clinical neurological applications.
These technological advances enable the system to achieve ultrafine 0.2-mm in-plane anatomical resolution, which has the potential to enable visualization of anatomical structures previously unseen on MRI, such as clinical details in cortical structure. Terra also includes two coils optimized for clinical neuro and knee imaging.



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)




.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)











