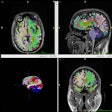
The U.S. Food and Drug Administration (FDA) has issued a safety alert regarding the reliability of MR thermometry with MRI-guided laser interstitial thermal therapy (MRgLITT) devices.
MRgLITT devices are used in neurosurgical procedures for the ablation of brain tumors, epileptic foci, or radiation necrosis. Changes in temperature at the treatment site are monitored with MR thermometry.
The agency has reviewed medical device and literature reports that describe adverse events when these devices were used to treat intracranial lesions. It is evaluating data that suggest potentially inaccurate MR thermometry information can be displayed during treatment, and that MRgLITT devices may not account for the continued thermal spread of energy to the surrounding tissue, which may result in an underestimation of thermal damage.
The FDA recommends that healthcare providers discuss with patients the benefits and risks of these devices. More information is available on the FDA's website.



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)